FDA Approves Madrigal's Rezdiffra™ for Moderate to Severe Noncirrhotic NASH
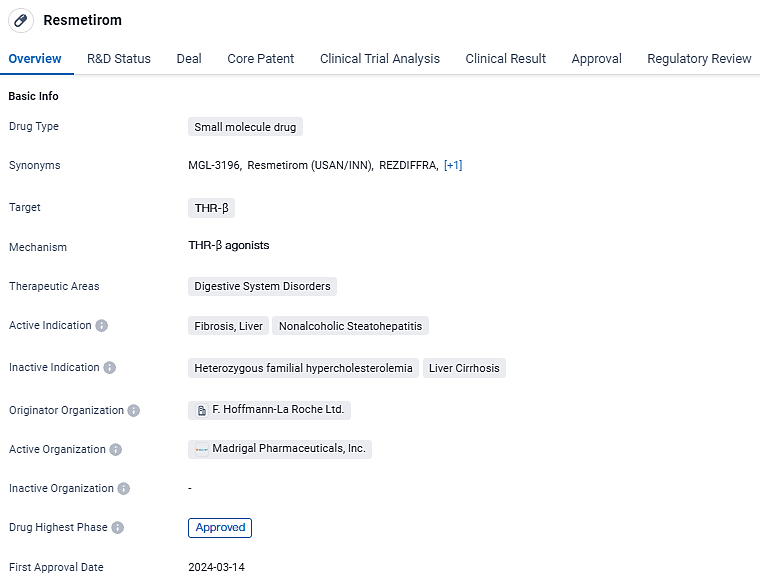
Madrigal Pharmaceuticals, Inc., has disclosed that the U.S. Food and Drug Administration provided provisional authorization for the drug Rezdiffra (resmetirom) to be used together with dietary and physical activity regimen to manage noncirrhotic NASH in adults experiencing moderate to severe hepatic fibrosis. This expedited endorsement might hinge upon future substantiation and detailed reporting of therapeutic effects as derived from currently progressing trials meant to corroborate these findings.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Madrigal's CEO Bill Sibold expressed, "NASH, characterized by moderate to severe liver fibrosis, represents a critical and escalating health concern that has lacked an FDA-sanctioned treatment solution. The present milestone is significant for the domain of NASH and exemplifies the pinnacle of achievement in our sector. We are thrilled to be able to offer Rezdiffra to those who are desperately in need."
Dr. Becky Taub, a principal architect alongside serving as Madrigal's Chief Medical Officer and R&D President, mentioned, "Our sincere gratitude extends to the multitude of participants in our clinical trials, whose involvement was pivotal for the swift sanction of Rezdiffra. Our conviction is firm that Rezdiffra is set to revolutionize the management approach for NASH with elevated degrees of liver fibrosis, granting medical practitioners a powerful therapy directed at enhancing fibrosis and resolving NASH, averting the progression to cirrhosis."
Wayne Eskridge of the Fatty Liver Foundation, who co-founded and leads the organization, remarked, "Today marks a jubilant occasion for NASH patients, for whom the path to the inaugural licensed therapy has been long. This landmark authorization injects renewed vigor into the NASH community, fostering a surge in education about the disease, refinement of care methodologies, and magnified funding for NASH research."
The MAESTRO-NASH clinical trial is continuing as it seeks to acquire confirmatory outcome data, which, if affirmative, will corroborate the therapeutic advantage of Rezdiffra and possibly bolster its full endorsement. Another concurrent trial is probing the potential delay of liver decompensation occurrences in patients with NASH cirrhosis under stable conditions and administered with Rezdiffra, in comparison to a placebo.
Patients in the United States can anticipate Rezdiffra becoming accessible by April, distributed exclusively via a select network of specialty pharmacies. Madrigal is dedicated to ensuring that qualifying patients are able to procure Rezdiffra, facilitated by the Madrigal Patient Support initiative. This service is structured to assist in overcoming barriers such as insurance and financial concerns and to provide co-pay aid to qualifying individuals. Additionally, Madrigal has developed a program to assist uninsured patients in acquiring Rezdiffra.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
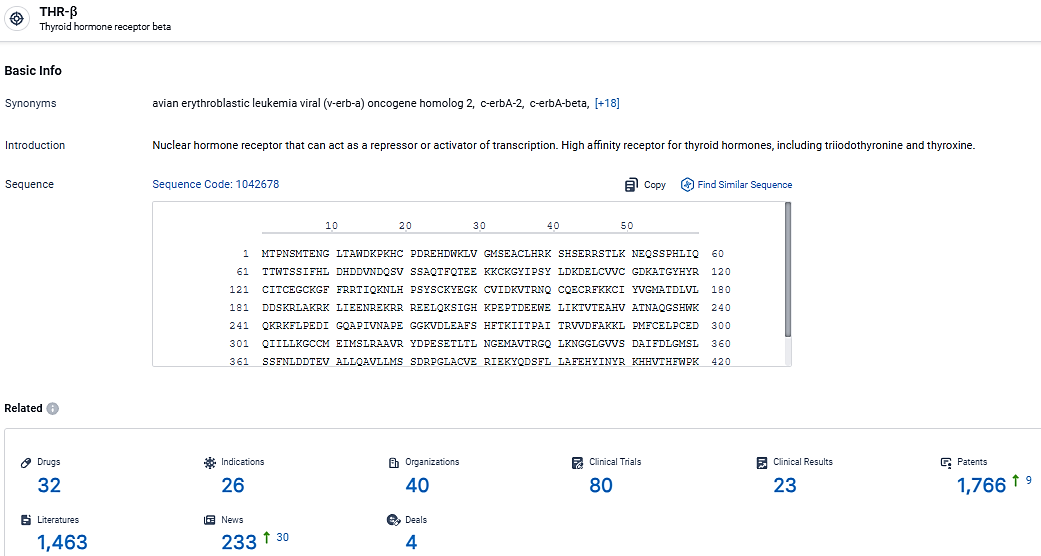
According to the data provided by the Synapse Database, As of March 17, 2024, there are 32 investigational drugs for the THR-β target, including 26 indications, 40 R&D institutions involved, with related clinical trials reaching 80, and as many as 1766 patents.
Resmetirom targets THR-β and is indicated for the treatment of fibrosis and liver nonalcoholic steatohepatitis. With its recent approval in the United States and breakthrough therapy designation, Resmetirom shows promise in addressing unmet medical needs and improving outcomes for patients with these conditions.