FDA Approves Rezolute's Phase 3 Study of RZ358 for Tumor-Induced Hypoglycemia
Rezolute, Inc., an advanced-stage biopharmaceutical firm focused on creating innovative and transformative treatments for severe rare illnesses, declared that it has obtained approval from the U.S. Food and Drug Administration for its Investigational New Drug application regarding RZ358 (ersodetug) aimed at addressing hypoglycemia in individuals suffering from tumor-induced hyperinsulinism.
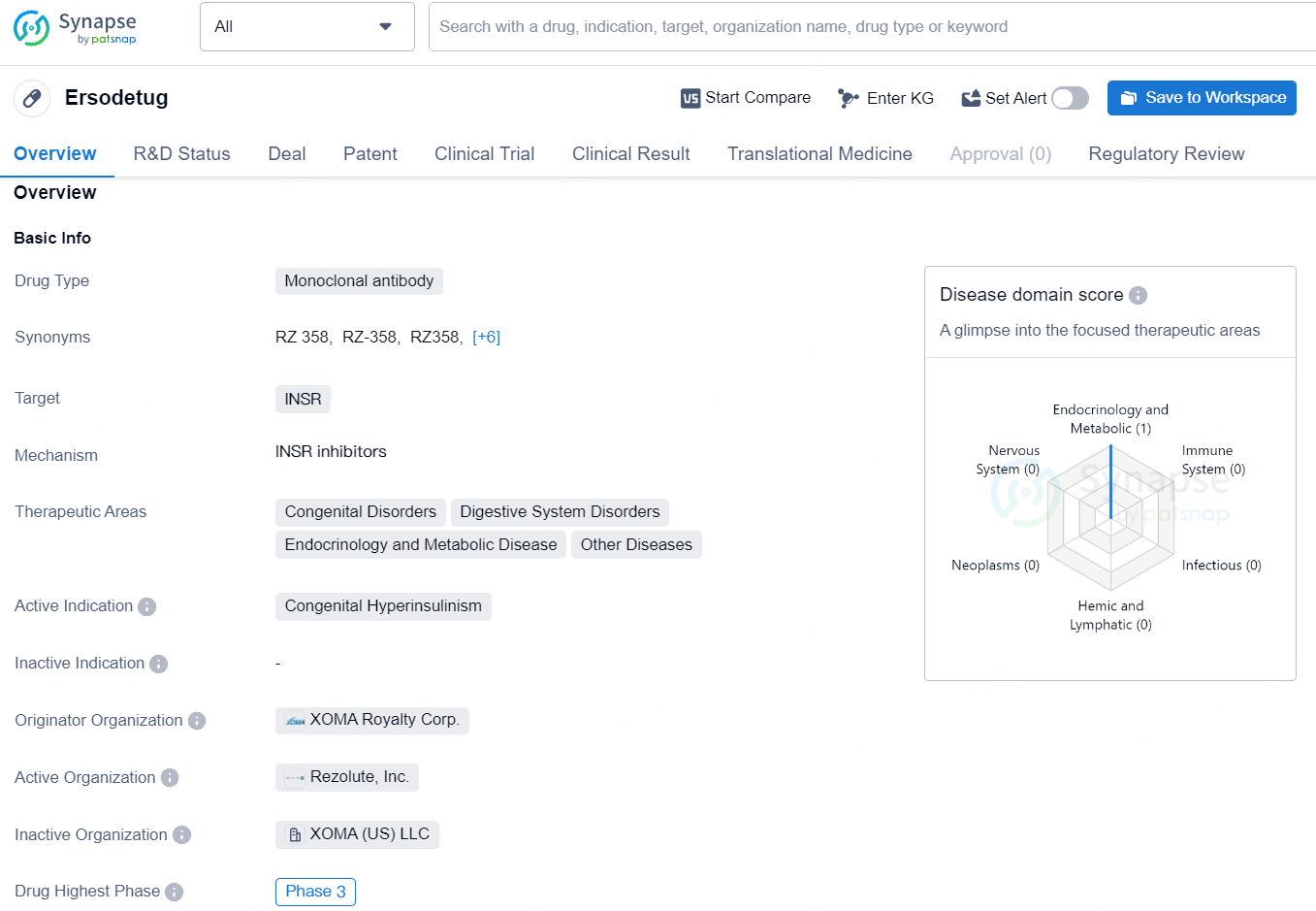
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The organization is embarking on the initial startup phases for the study, primarily to be conducted in the United States, with patient enrollment anticipated to begin in the first half of 2025. Ersodetug is also under investigation in an ongoing global Phase 3 clinical trial focused on patients with congenital HI. The primary data from this trial is expected to be available by mid-2025.
“Hypoglycemia related to tumor HI necessitates treatment to avert severe adverse effects and to enhance patients’ daily functioning and quality of life, including making it feasible for them to undergo tumor-targeted therapies,” commented Brian Roberts, M.D., Chief Medical Officer at Rezolute.
“We are optimistic about the significant real-world benefits we have observed in tumor HI patients who have previously used ersodetug in our Expanded Access Program, alongside the safety and efficacy shown in clinical trials for patients with congenital HI, a related condition. We believe that the FDA’s clearance of our IND for this Phase 3 study acknowledges the potential of ersodetug to address this critical unmet need, and we are thrilled to be advancing one step closer to a potential universal treatment for hypoglycemia induced by all forms of HI,” Roberts added.
Ersodetug is a fully human monoclonal antibody that targets an allosteric site on the insulin receptor in tissues such as the liver, fat, and muscle. This action counteracts the excessive activation of the insulin receptor by insulin and similar hormones, thereby correcting hypoglycemia. Ersodetug has the potential to be a universally effective treatment for hypoglycemia caused by both congenital and acquired forms of HI.
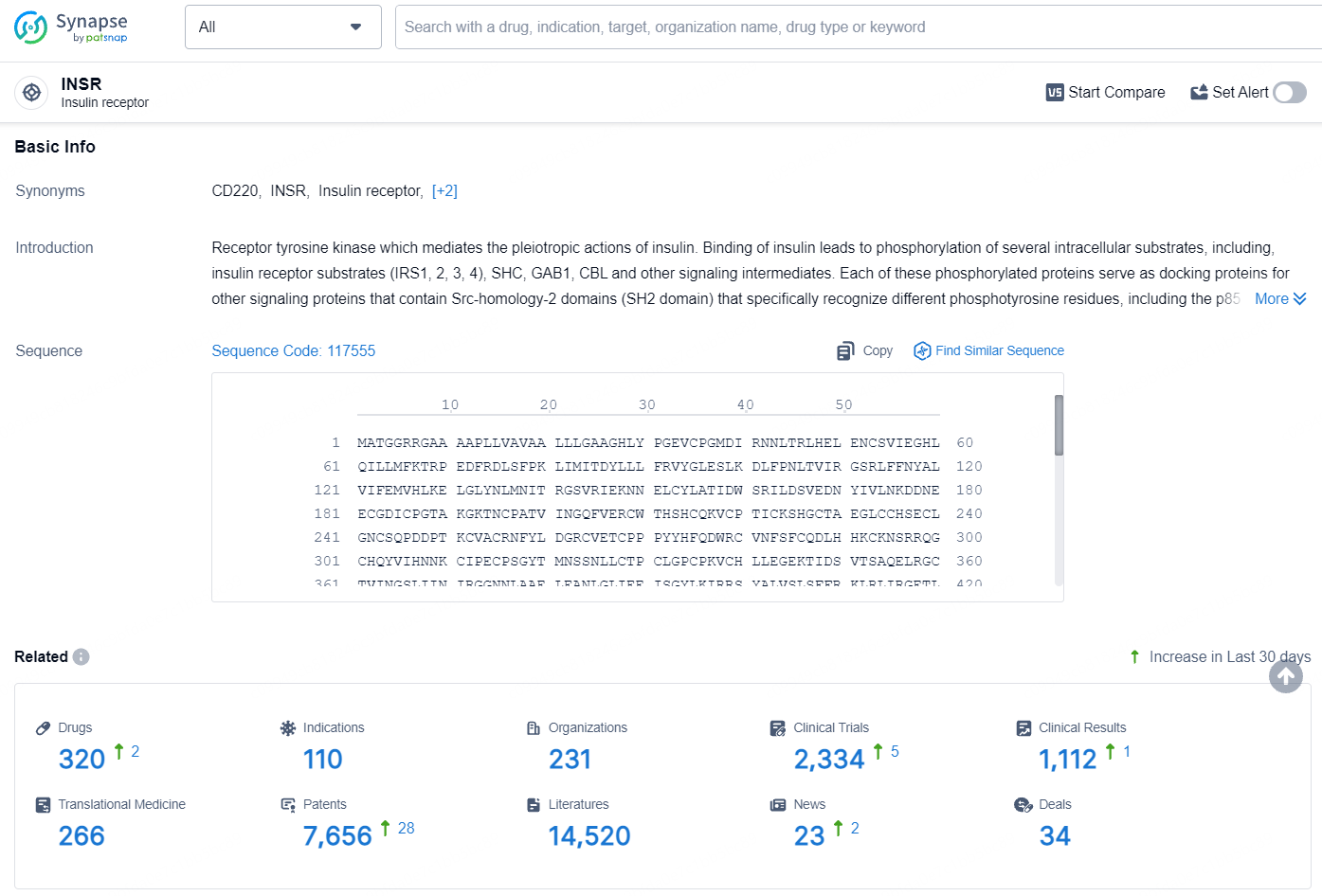
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 8, 2024, there are 320 investigational drugs for the insulin receptor target, including 110 indications, 231 R&D institutions involved, with related clinical trials reaching 2334, and as many as 7656 patents.
Ersodetug is a promising monoclonal antibody drug targeting the insulin receptor for the treatment of Congenital Hyperinsulinism. Its advancement to Phase 3, along with regulatory designations for rare pediatric disease and orphan drug status, underscores its potential significance in addressing this rare medical condition. The drug's development by XOMA Royalty Corp. further positions it as a key player in the field of biomedicine, with the potential to make a significant impact on the treatment of Congenital Hyperinsulinism.