Imbrium Therapeutics Files Application to Study Sunobinop for Alcohol Use Disorder Treatment
Imbrium Therapeutics L.P., a branch of Purdue Pharma L.P., has filed an Investigational New Drug Application with the U.S. Food and Drug Administration to assess the use of sunobinop for potentially treating moderate to severe alcohol use disorder.
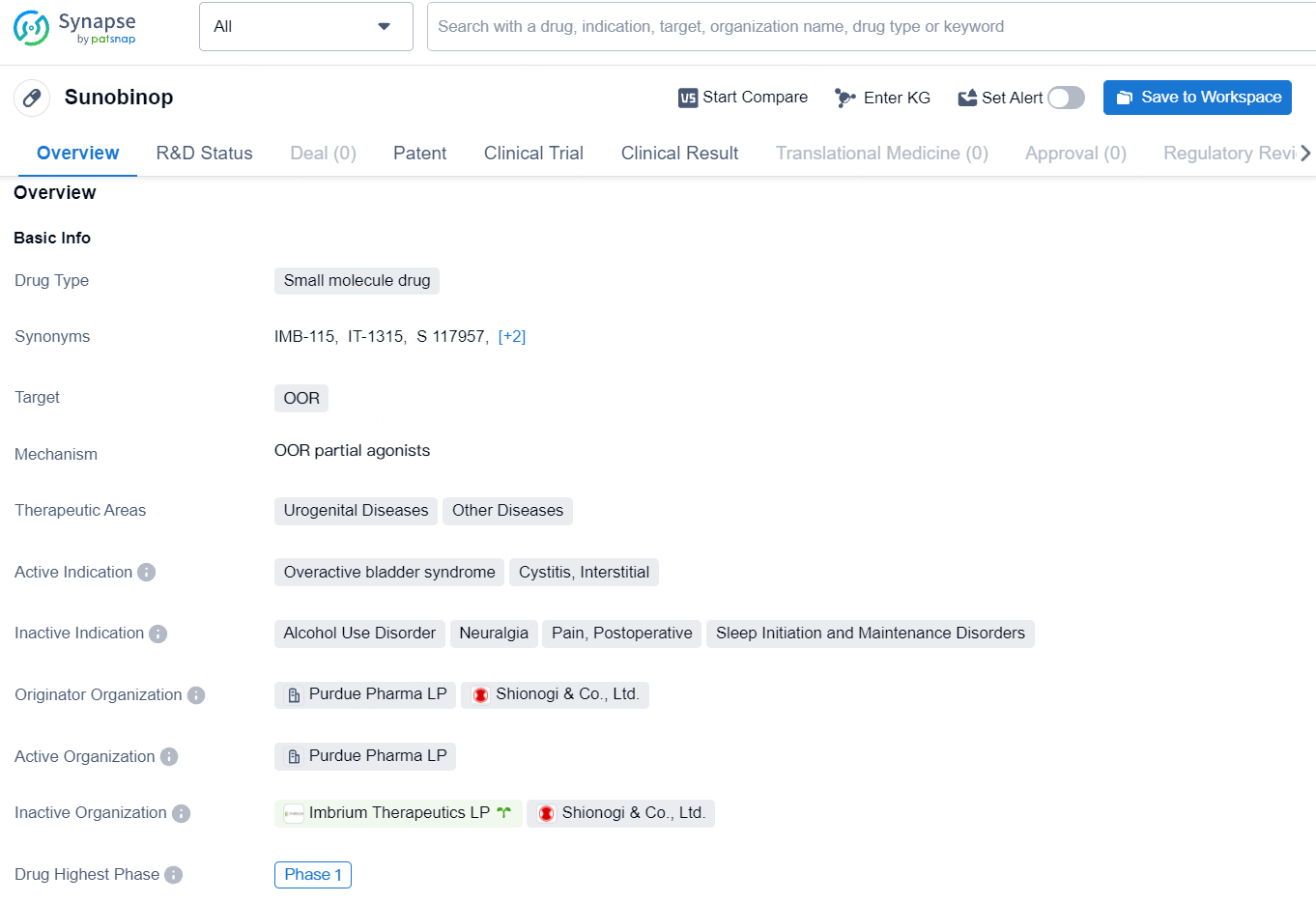
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Sunobinop is an investigational, innovative, and possible first-in-class oral compound discovered by our researchers that is currently under clinical trials. Sunobinop is designed to interact with and activate the nociceptin/orphanin-FQ peptide receptor (NOP), a protein significantly expressed in the central and peripheral nervous system, impacting various biological processes.
"Over recent years, the data we have gathered in our sunobinop development project has supported this potent, partial, and selective agonist as a potential treatment option for AUD and other serious conditions," stated Dr. Julie Ducharme, Vice President, Chief Scientific Officer.
"As a pharmaceutical company committed to R&D and public health improvement, we eagerly anticipate expanding our understanding of sunobinop and the role of NOP receptors in AUD," stated Craig Landau, MD, President and CEO, Purdue.
Imbrium is assessing sunobinop as a potential treatment for AUD, as well as for overactive bladder syndrome and interstitial cystitis/bladder pain syndrome. This IND submission is based on data that suggest NOP agonists, such as sunobinop, could be effective in the treatment of AUD.
Preclinical studies have demonstrated that NOP activation in AUD models diminishes the reinforcing and motivating effects of ethanol. In human studies, NOP activity has been linked to relapse risk in AUD patients.
The National Institute of Drug Abuse has identified NOP receptor agonists as one of the top ten pharmacological mechanisms for the rapid development of therapeutics aimed at treating substance use disorder.
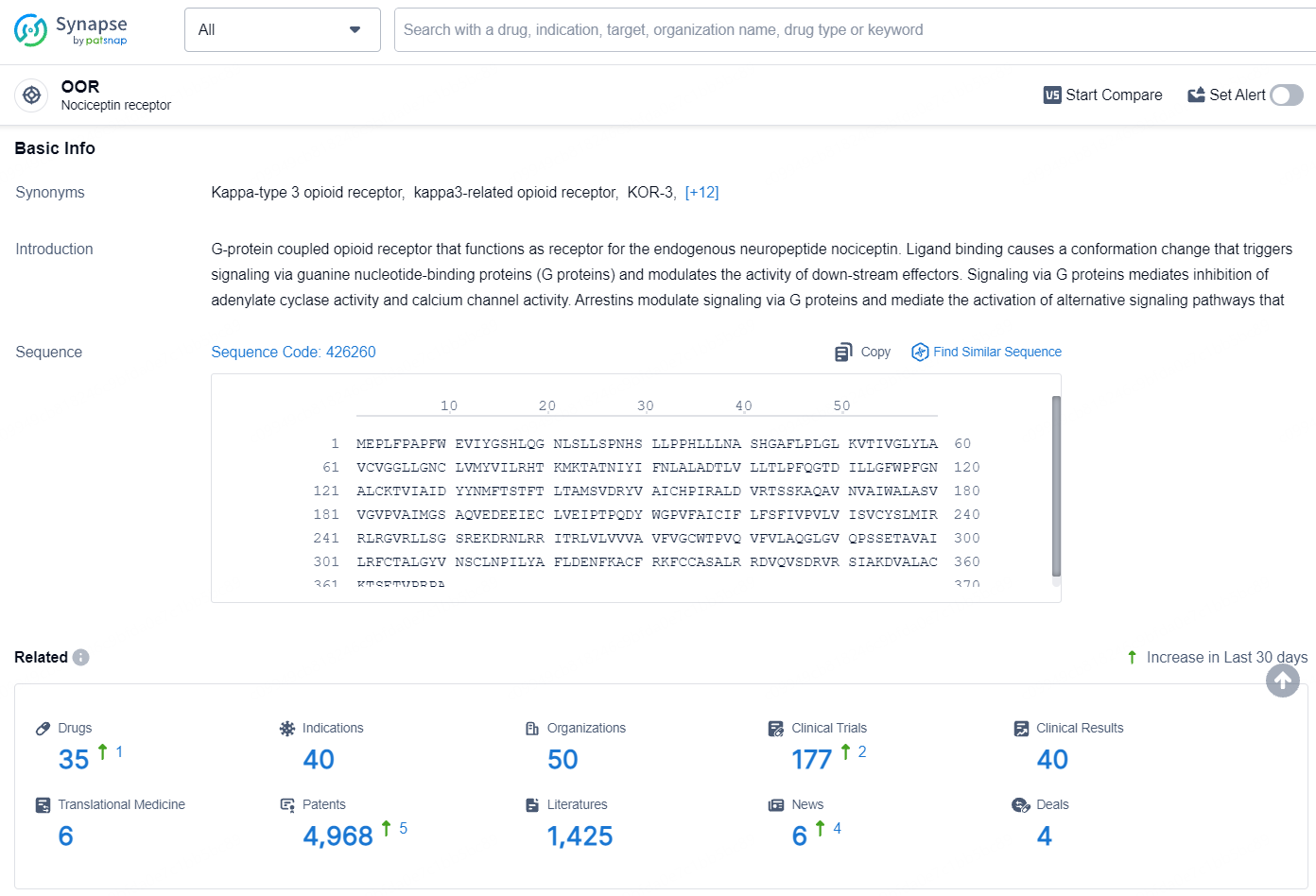
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 8, 2024, there are 35 investigational drugs for the OOR target, including 40 indications, 50 R&D institutions involved, with related clinical trials reaching 177, and as many as 4968 patents.
Sunobinop is a small molecule drug designed to target OOR, and it is currently in the highest phase of global development, which is Phase 1. Sunobinop represents a promising development in the pharmaceutical industry, with the potential to address unmet medical needs in the treatment of urogenital diseases and other related conditions. As it continues to progress through clinical development, further insights into its potential therapeutic benefits and commercial prospects may become available.