FDA Approves Vertex's Clinical Trials for ADPKD Drug VX-407
Vertex Pharmaceuticals Incorporated has disclosed that the U.S. FDA has given approval to begin exploring the potential of VX-407. This groundbreaking new chemical entity is being developed as a novel treatment designed to directly address the root genetic mechanisms involved in autosomal dominant polycystic kidney disease, with a focus on a specific range of PKD1 genetic mutations.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Autosomal Dominant Polycystic Kidney Disease (ADPKD) stands as the predominant genetic renal disorder, impacting approximately a quarter of a million individuals across the United States and Europe. Despite its prevalence, there remains a lack of available therapies that effectively tackle the fundamental biological origins of this condition.
Marked by the development of multiple cysts that expand within the kidneys, ADPKD progressively deteriorates renal functions, potentially culminating in renal failure which necessitates either dialysis or kidney transplant interventions, and often results in decreased life expectancy.
The genesis of most cases of ADPKD can be traced back to mutations in the PKD1 gene, responsible for the synthesis of the polycystin 1 protein. The genetic alterations result in diminished functionality of PC1, precipitating the formation of cysts. VX-407 emerges as an innovative therapeutic small molecule, conceived to specifically rectify the root cause of ADPKD for a fraction of those affected by PKD1 gene variants. This population is roughly estimated to be around 10% of the total ADPKD patient group, equating to roughly 25,000 patients. Vertex is on track to debut a Phase 1 clinical trial for VX-407, starting with healthy participants, within the current month.
Carmen Bozic, M.D., Executive Vice President for Global Medicines Development and Medical Affairs, as well as Chief Medical Officer at Vertex, expressed that the progression of VX-407 into clinical testing signifies a pivotal step towards revolutionizing the management of this critical illness. Bozic underscored the company's commitment to continuous innovation, analogous to their strategy in addressing cystic fibrosis, with the aspiration to eventually support all 250,000 individuals afflicted with ADPKD.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
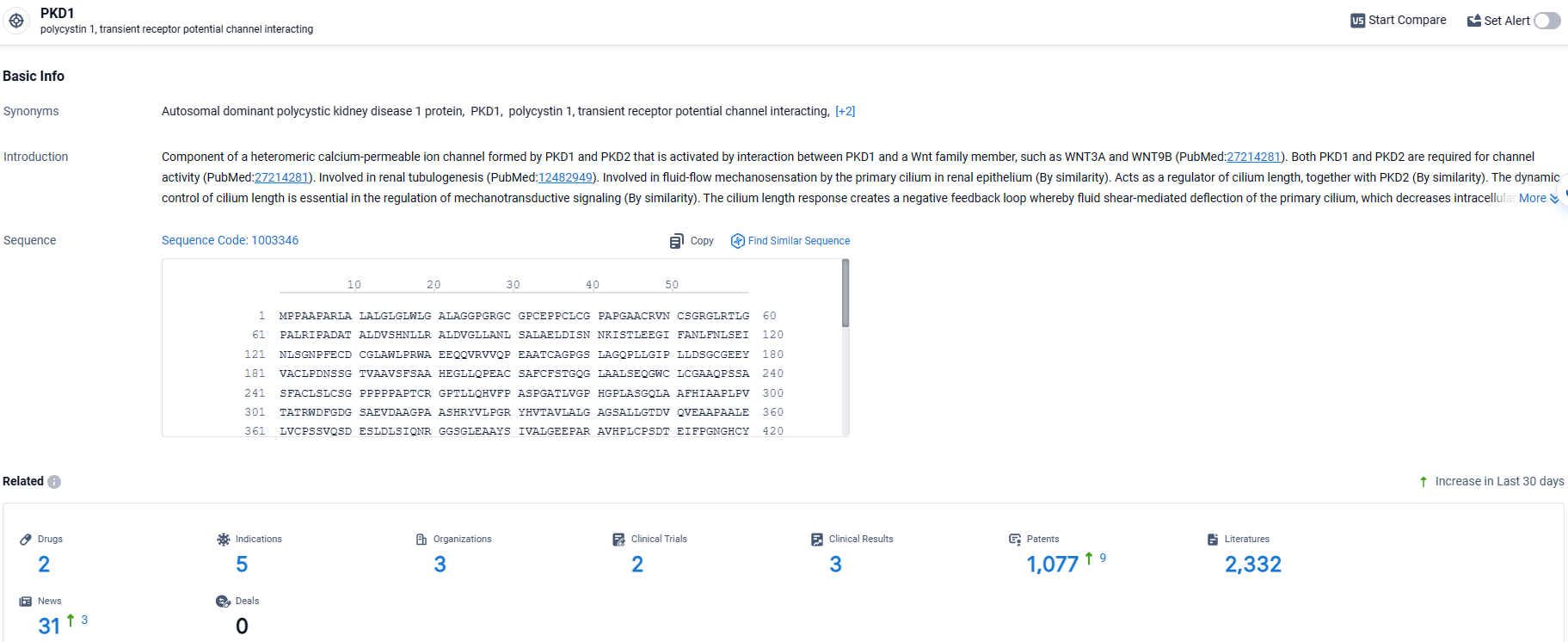
According to the data provided by the Synapse Database, As of March 25, 2024, there are 2 investigational drugs for the PKD1 target, including 5 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 1077 patents.
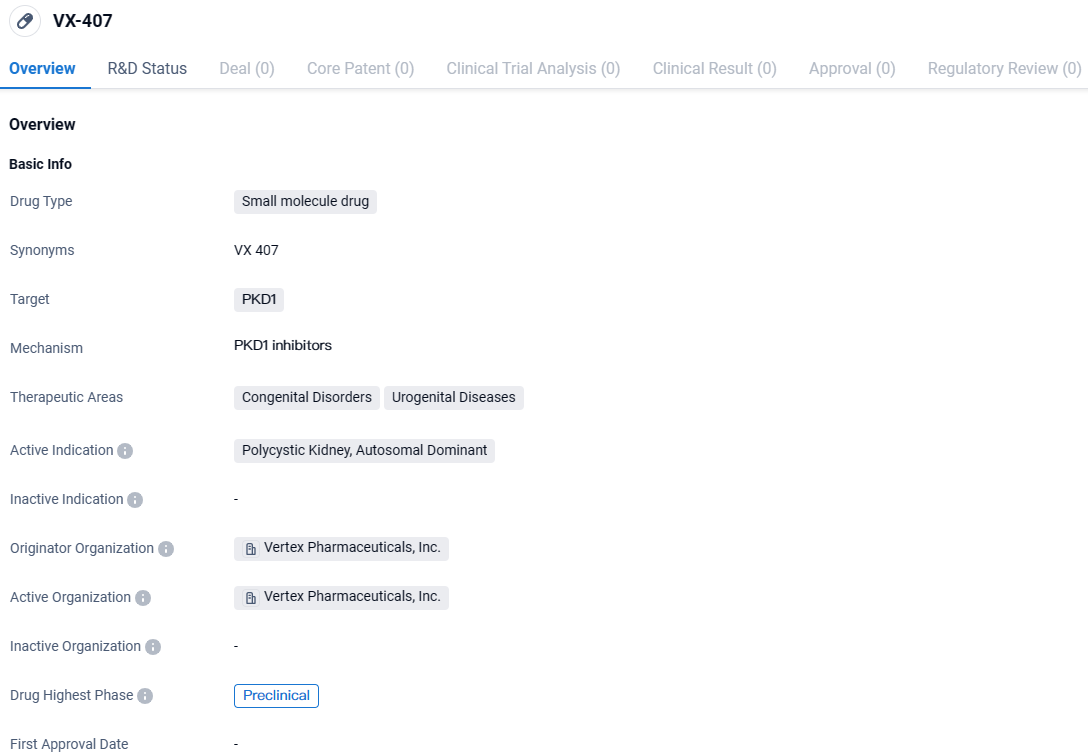
VX-407 targets PKD1 and is intended for the treatment of Polycystic Kidney, Autosomal Dominant. Currently in the preclinical phase, VX-407 shows promise in potentially addressing an unmet medical need in the field of congenital disorders and urogenital diseases. Further research and clinical trials will be necessary to determine its safety and efficacy in humans.