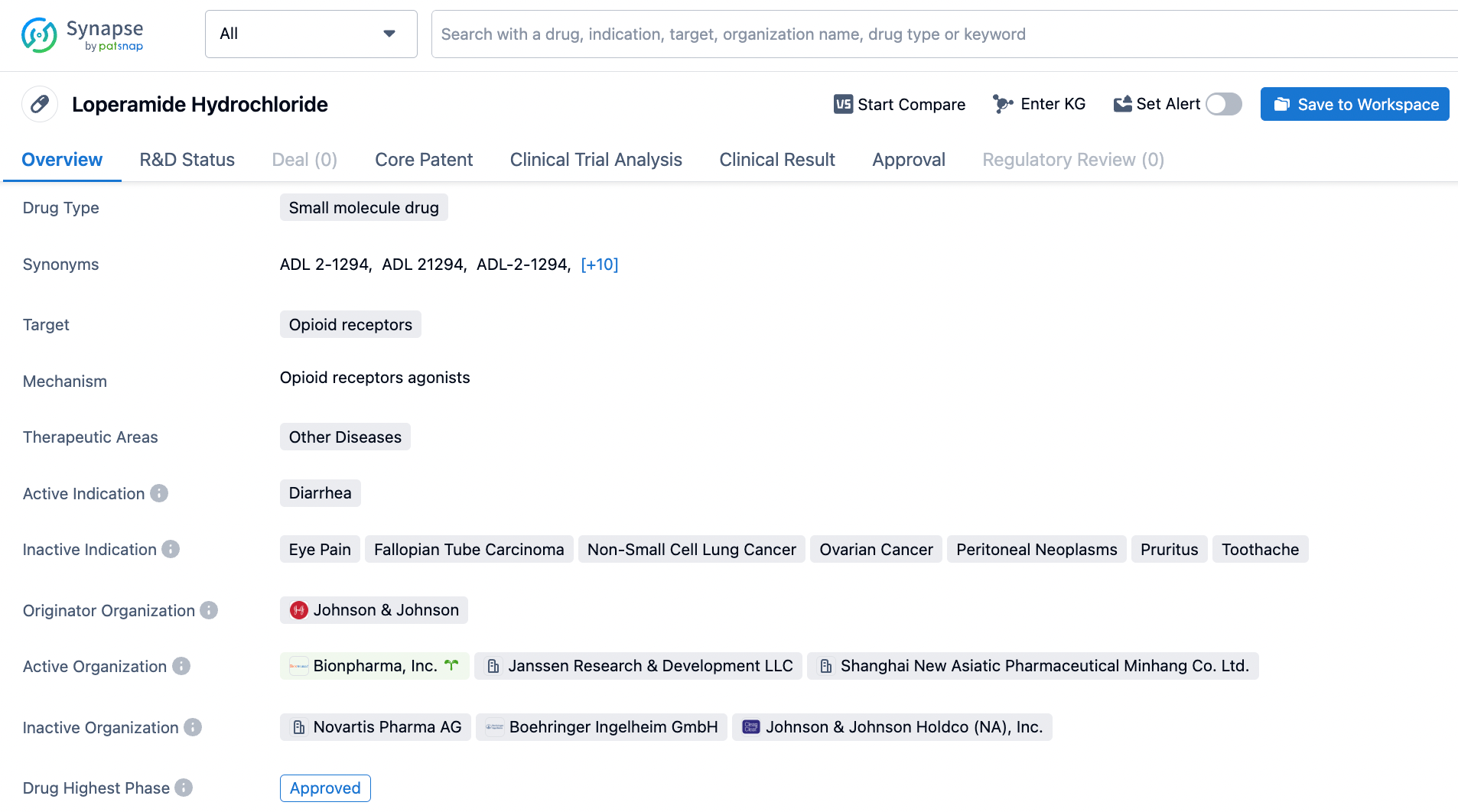
Master Loperamide Search on Synapse
Loperamide, an FDA-approved small molecule drug since December 1976, is a puzzling substance that binds to opioid receptors, specifically the mu, kappa, and delta receptors, and acts as an agonist, to reduce the frequency and urgency of bowel movements by slowing down the movement of the intestines. Diarrhea is the main indication of this drug, which is administered to alleviate symptoms associated with this condition. Johnson & Johnson is the originator organization of Loperamide, and the drug has been used widely in clinical practice for decades. Although Loperamide may cause side effects, including constipation and abdominal cramping, it remains an important tool in the treatment of diarrhea. Nonetheless, it is worth noting that Loperamide should not be utilized in cases of fever or bloody or mucus-filled stools as they could indicate more severe underlying conditions. Click on the image below to begin the exploration journey of Loperamide through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!