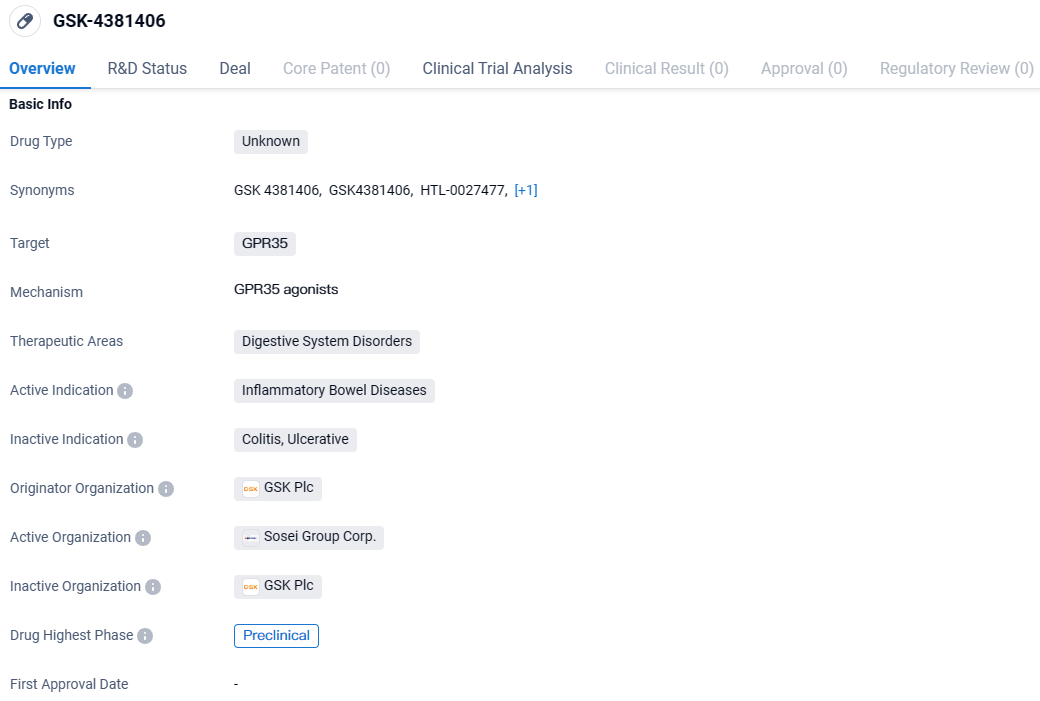
Sosei Heptares Regains Full Rights to Oral GPR35 Activator HTL0027477 from GSK for Inflammatory Bowel Trials
Sosei Group Corporation has declared that it has reacquired complete control over HTL0027477 (formerly GSK4381406), a development-stage, orally administered GPR35 agonist with high selectivity and novel properties, intended as an innovative therapy option for Inflammatory Bowel Diseases. This compound is poised to enter clinical trials.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
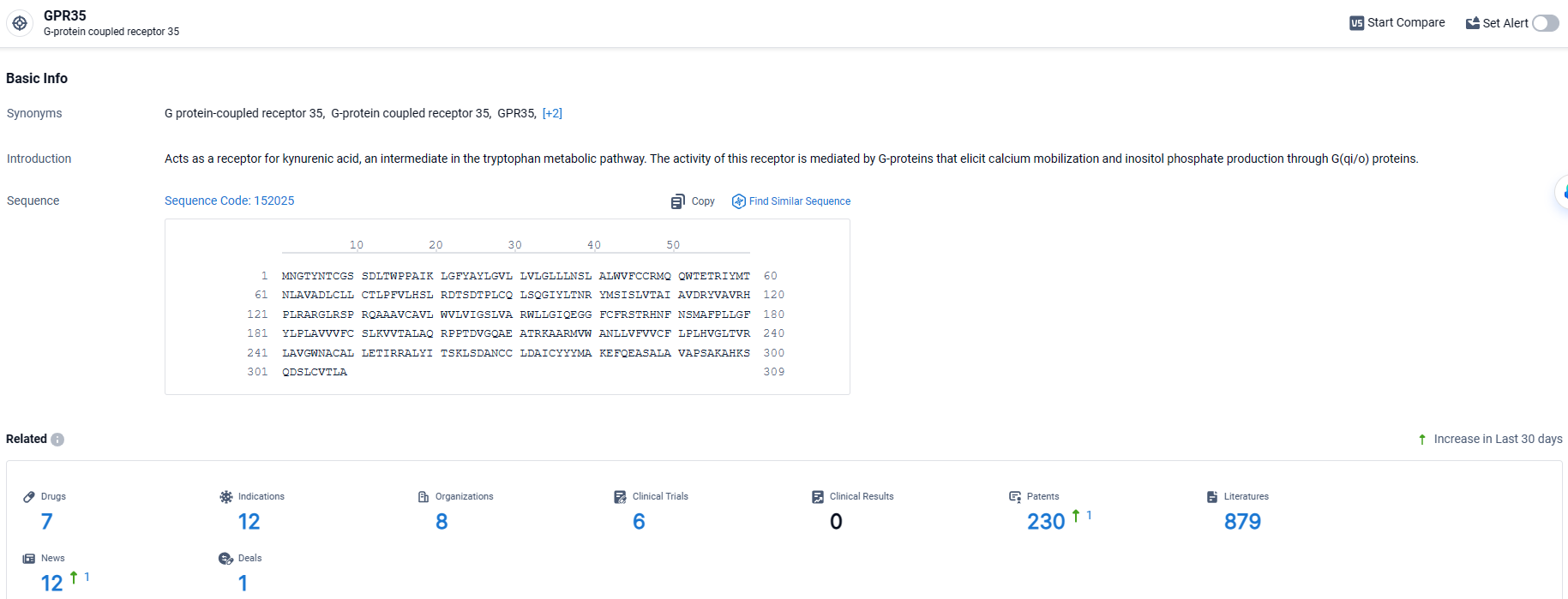
GPR35 stands out as a key orphan receptor within the G protein-coupled receptor (GPCR) family, closely linked to inflammatory bowel disease (IBD), a condition that still presents a substantial care gap affecting millions globally.
Conceived by Sosei Heptares through its unique structure-based drug design technology, HTL0027477 was subsequently transferred to GSK in 2020. Progressing through a collaborative development venture, this compound has shown encouraging results in terms of mechanism, preclinical effectiveness, and safety profiles. These findings support its potential use in managing gut barrier integrity and diminishing gastrointestinal discomfort in conditions like ulcerative colitis and irritable bowel syndrome.
In mid-2023, regulatory clearance from the UK Medicines and Healthcare products Regulatory Agency was granted for the exploration of HTL0027477 in preliminary human trials.
Late in 2023, steps were taken to recapture the rights to this venture after GSK's decision to scale back on its advancement due to a revised outlook on immunology research and a shift in its research leadership. This decision did not stem from any concerns regarding the scientific, preclinical, or safety data pertaining to the drug candidate.
Subsequent to GSK's strategic move, Sosei Heptares reclaimed complete control over the HTL0027477 initiative, including all related intellectual property previously granted to GSK, and all preclinical findings produced from their alliance, all without an initial fee. Moving forward, Sosei Heptares aims to map out the most effective route for the future clinical progression of this program, which may involve direct development or seeking new partnership arrangements.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 25 2024, there are 7 investigational drugs for the GPR35 target, including 12 indications, 8 R&D institutions involved, with related clinical trials reaching 6, and as many as 230 patents.
GSK-4381406 targets GPR35 and focusing on digestive system disorders, particularly inflammatory bowel diseases. While the drug is currently in the preclinical phase, its potential to address the chronic inflammation associated with these conditions makes it an interesting candidate for further research and development.