FDA Approves YORVIPATH® as First Treatment for Adult Hypoparathyroidism
Ascendis Pharma A/S (Nasdaq: ASND) has announced that the U.S. Food & Drug Administration (FDA) has given the green light for YORVIPATH® (palopegteriparatide; also known as TransCon PTH) to treat adult hypoparathyroidism. YORVIPATH is a prodrug of parathyroid hormone (PTH[1-34]), which is taken once daily and formulated to ensure continuous PTH release throughout a 24-hour period. Hypoparathyroidism, a rare endocrine disorder characterized by inadequate parathyroid hormone levels, influences various organs and affects an estimated 70,000 to 90,000 individuals in the United States.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Jan Mikkelsen, Ascendis Pharma’s President and CEO, stated, “Our second TransCon product, YORVIPATH, receiving FDA approval underscores our dedication to science and patient care, highlighting our rigorous commitment over the years to fulfilling the significant unmet medical demands of the hypoparathyroidism community in the U.S. We express our profound gratitude to patients, clinicians, and advocates whose contributions made this milestone possible.” Upon release, Ascendis will provide a comprehensive suite of patient services for YORVIPATH through its U.S. Ascendis Signature Access Program (A.S.A.P.), offering support throughout the treatment process and financial assistance for qualifying patients.
Patty Keating, Executive Director of the HypoPARAthyroidism Association, remarked, “The FDA approval of YORVIPATH is a pivotal achievement for our community. We are thankful that the severity of our condition has been acknowledged and our voices recognized. We eagerly anticipate this new treatment to help alleviate the limitations and dangers associated with conventional therapies.”
The FDA’s approval of YORVIPATH is based on the evaluation of the clinical package for TransCon PTH (palopegteriparatide) that was submitted with the Company’s New Drug Application, which included data from the global Phase 2 PaTH Forward and Phase 3 PaTHway trials.
Lynn Kohlmeier, M.D., an endocrinologist at Spokane Osteoporosis & Endocrinology, Chair of the Medical Advisory Board of the HypoPARAthyroidism Association, and PaTHway Trial investigator, commented, “Hypoparathyroidism's impact on patient health and quality of life can be profoundly debilitating. Addressing the root cause is vital and represents a significant advancement for our patients.”
Ascendis is finalizing manufacturing for the U.S. market and expects the initial supply of YORVIPATH to be available in Q1 2025. Additionally, Ascendis plans to seek FDA approval to market the already manufactured product, potentially making it available in the U.S. by Q4 2024.
Hypoparathyroidism is an endocrine disorder characterized by insufficient production of parathyroid hormone (PTH), which regulates calcium and phosphate homeostasis in the body, acting on bones, kidneys, and intestines. Individuals affected by hypoparathyroidism are at risk for numerous severe and potentially life-threatening complications, such as neuromuscular irritability, kidney issues, extra-skeletal calcifications, and cognitive deficits. Post-surgical hypoparathyroidism accounts for the majority of cases (70-80%), with other causes including autoimmune and idiopathic origins.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
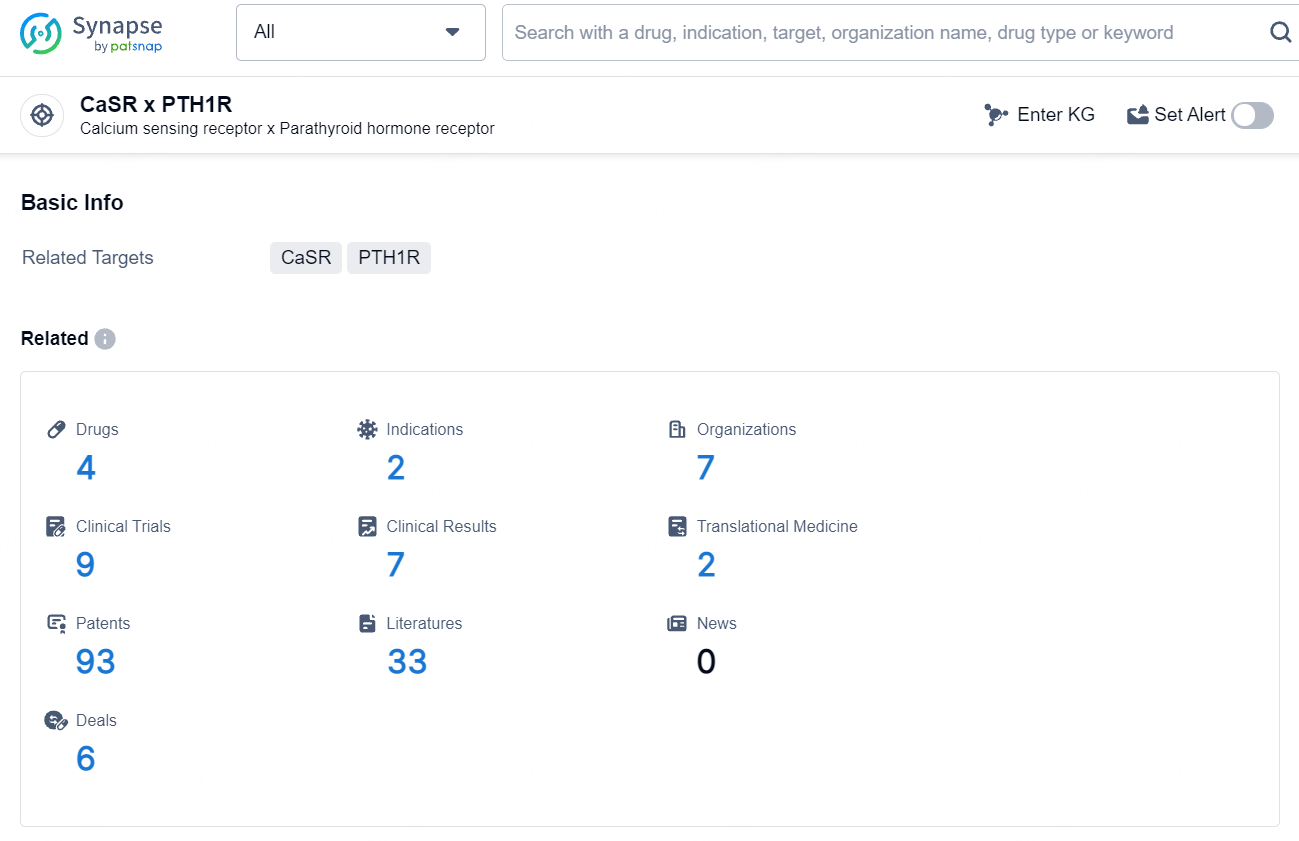
According to the data provided by the Synapse Database, As of August 14, 2024, there are 4 investigational drugs for the CaSR x PTH1R target, including 2 indications, 7 R&D institutions involved, with related clinical trials reaching 9, and as many as 93 patents.
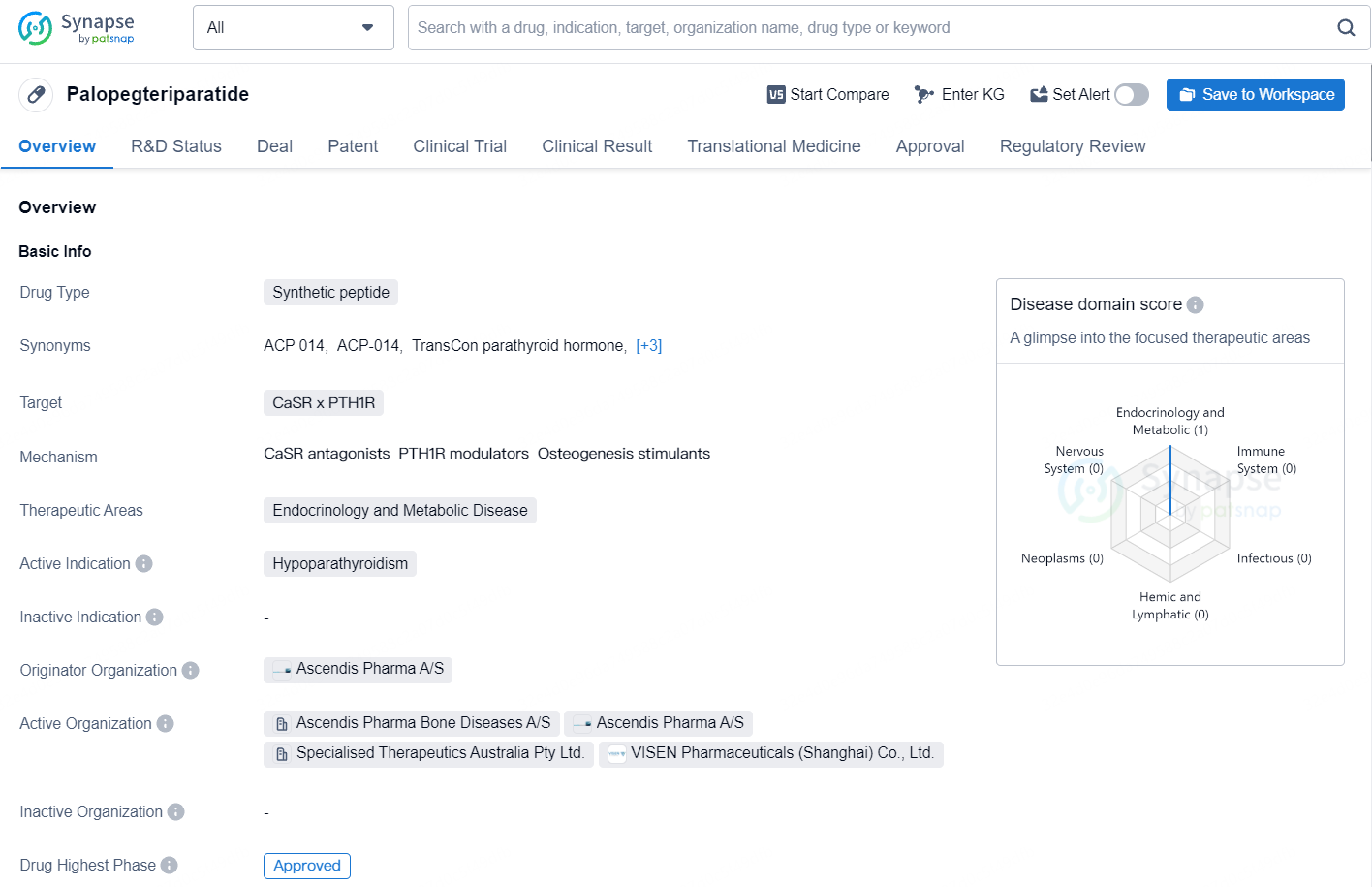
Palopegteriparatide is a synthetic peptide drug designed to target both the Calcium-Sensing Receptor (CaSR) and the Parathyroid Hormone Receptor 1 (PTH1R). It falls under the therapeutic areas of Endocrinology and Metabolic Disease, with its active indication being Hypoparathyroidism.