Merck Set to Purchase Experimental B-Cell Depletion Treatment, CN201, from Curon Biopharmaceutical
Merck (NYSE: MRK), also recognized as MSD outside of the U.S. and Canada, along with the private biotech firm Curon Biopharmaceutical (Curon), revealed today that they have concluded a definitive agreement. According to this deal, Merck, via one of its subsidiaries, will acquire CN201, an innovative investigational bispecific antibody currently in the clinical trial phase for treating B-cell related disorders.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dr. Dean Y. Li, president of Merck Research Laboratories, stated, “We are actively looking for ways to broaden and diversify our pipeline.” According to early clinical results, CN201 shows strong potential in targeting and reducing circulating and tissue B cells, which may help address a variety of malignant and autoimmune disorders.
Based on the established terms, Merck will, through a subsidiary, secure global rights to CN201 by making an initial payment of $700 million in cash. Curon is also slated to receive up to $600 million in milestone payments linked to the development and regulatory approval of CN201.
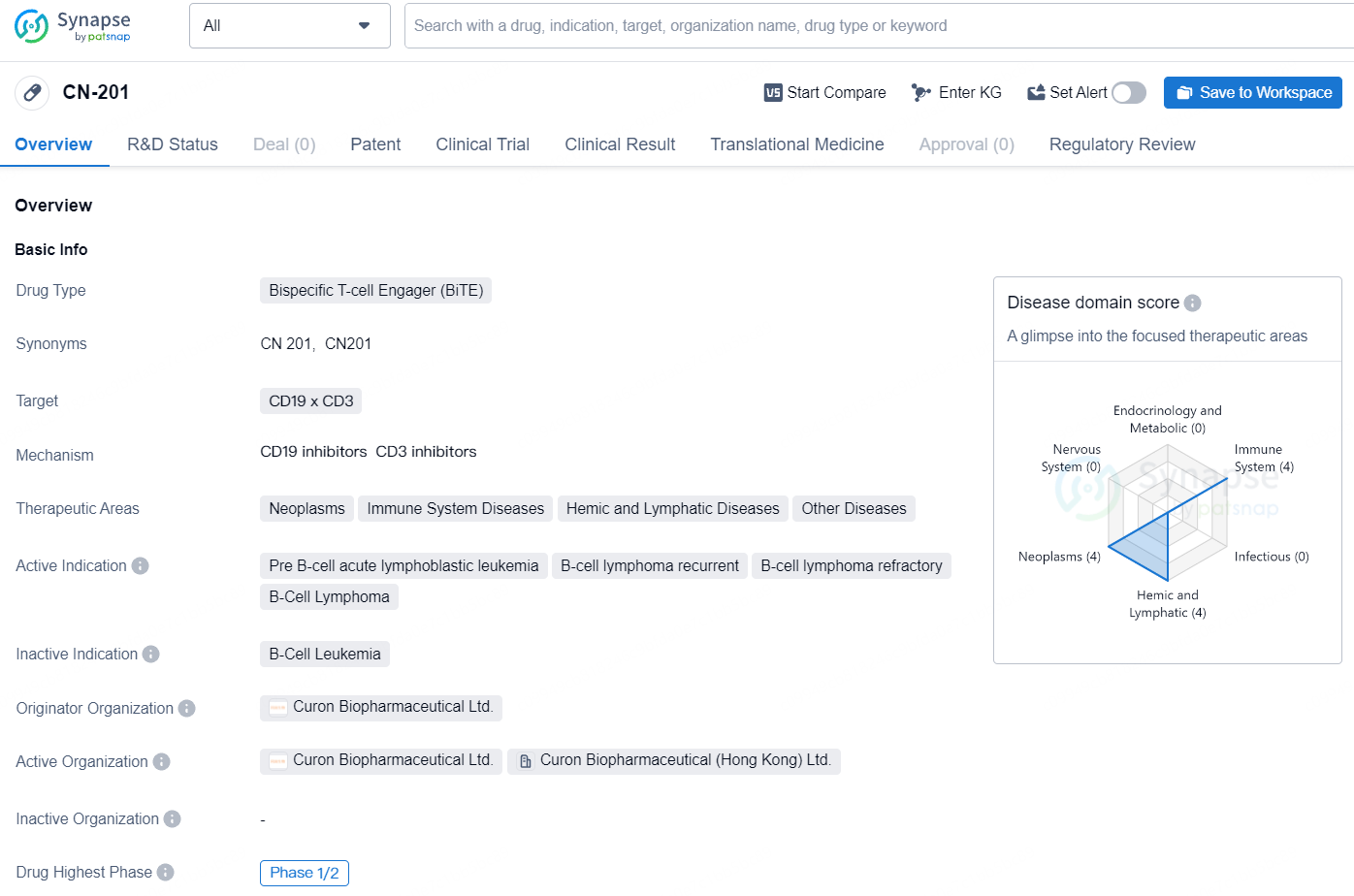
Currently, CN201 is undergoing Phase 1 and Phase 1b/2 clinical trials aimed at treating patients with relapsed or refractory non-Hodgkin’s lymphoma (NHL) and relapsed or refractory B-cell acute lymphocytic leukemia (ALL), respectively. Initial findings indicate that CN201 is active in patients with relapsed or refractory B-cell hematologic cancers and is well tolerated, potentially leading to significant and sustained decreases in B-cell populations. Merck intends to assess CN201 as a treatment option for B-cell malignancies and will also explore its potential as a novel, scalable therapy for autoimmune diseases.
“This agreement highlights the dedication and hard work of the Curon team,” commented Zhihong Chen, president and CEO of Curon. “As an innovator in immuno-oncology, Merck is uniquely positioned to expand upon our existing work and explore the comprehensive first-in-class potential of CN201.”
The completion of the proposed acquisition is subject to approval under the Hart-Scott-Rodino Antitrust Improvements Act and other standard conditions. The transaction is expected to be finalized in the third quarter of 2024 and will be accounted for as an asset acquisition. Merck anticipates recording a pre-tax charge of around $750 million (which includes the upfront payment and other related costs), equating to roughly $0.28 per share, in its non-GAAP results for the quarter when the transaction is concluded. Following its policy, Merck provides financial updates quarterly and will refresh its full-year financial outlook when announcing results for the third quarter of 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
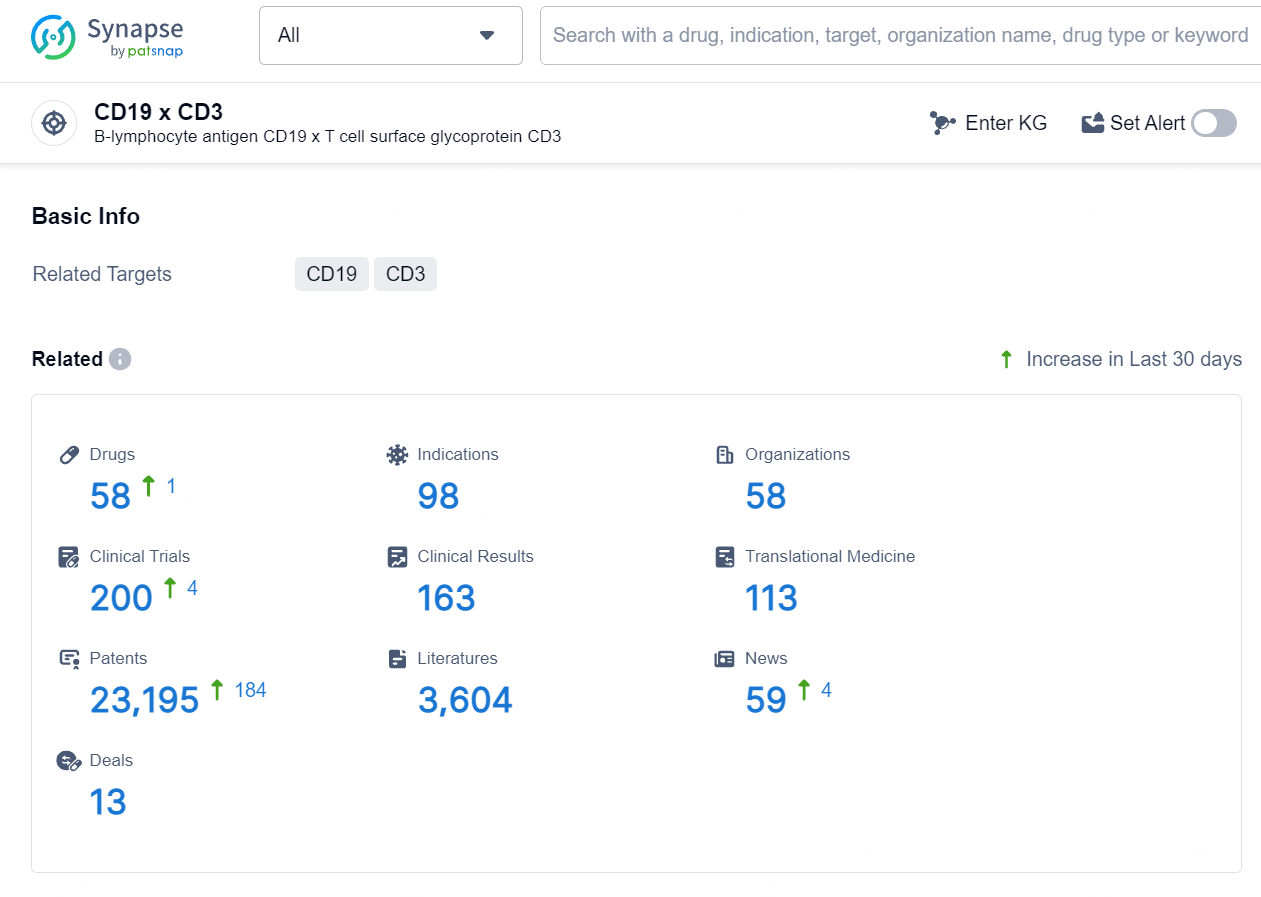
According to the data provided by the Synapse Database, As of August 12, 2024, there are 4 investigational drugs for the CD3xCD19 target, including 4 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 293 patents.
CN201 is a novel CD3xCD19-targeting T-cell-engager bispecific antibody, designed to target B cells for elimination by T cells. CN201 is currently being evaluated in Phase 1 and Phase 1b/2 clinical trials for the treatment of relapsed or refractory non-Hodgkin’s lymphoma and relapsed or refractory acute lymphocytic leukemia, respectively.