U.S FDA Approves IASO Bio's IND Application for Equecabtagene Autoleucel in Two More Autoimmune Disorders
IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical firm focused on the discovery, development, manufacture, and commercialization of cutting-edge cell therapy and antibody products, has announced that the U.S. Food and Drug Administration (FDA) has approved the investigational new drug (IND) application for its fully human anti-BCMA chimeric antigen receptor autologous T cell injection, known as Equecabtagene Autoleucel (Eque-cel). This approval is for the treatment of Non-renal Systemic Lupus Erythematosus (SLE) and Lupus Nephritis (LN). This represents the fifth IND approval for Eque-cel in the realm of autoimmune diseases across China and the United States.
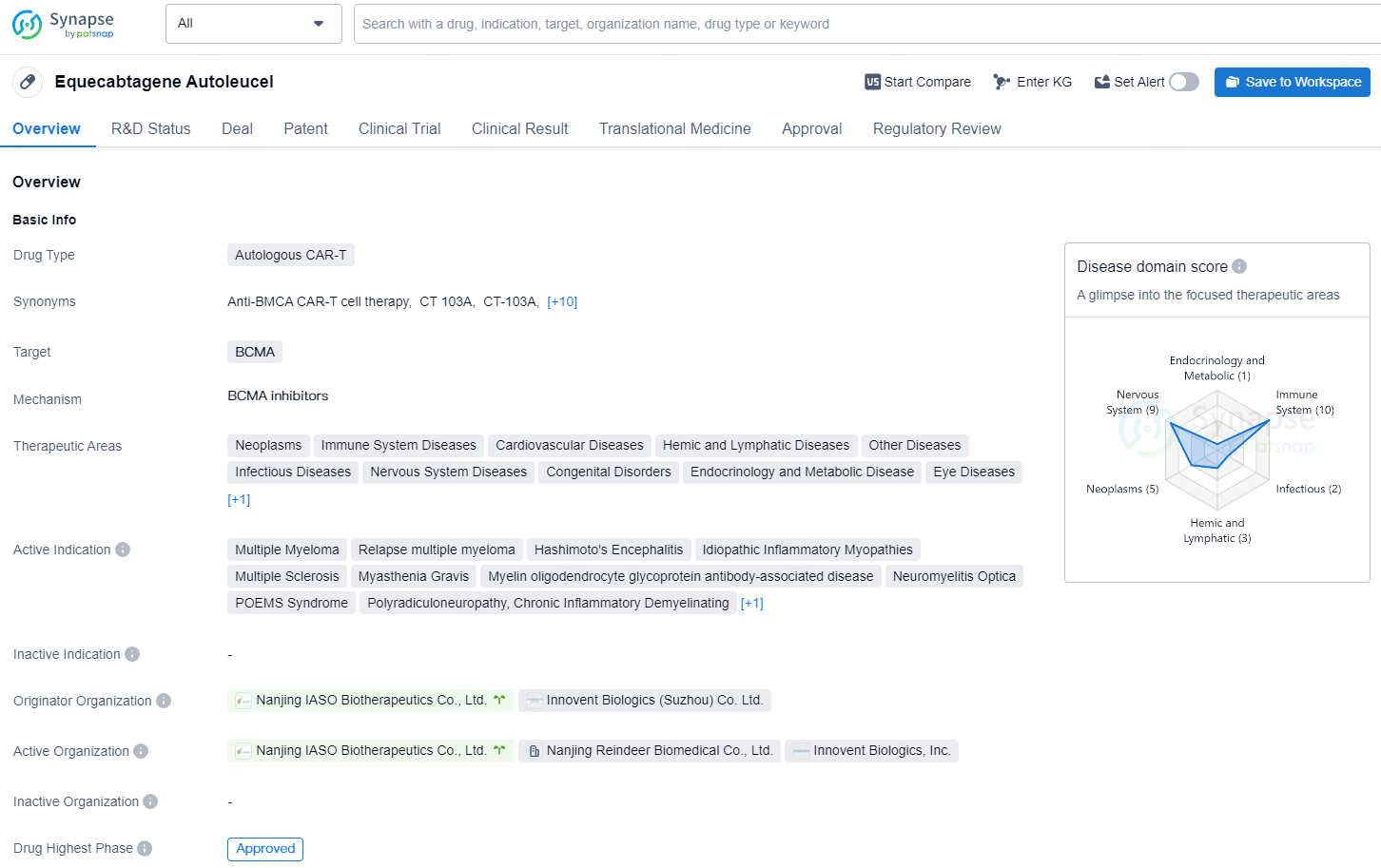
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"We're thrilled that the U.S. FDA has approved the IND for our two new indications—SLE and LN—further underscoring Eque-cel's potential in addressing autoimmune diseases," stated IASO Bio's Chief Scientific Officer, Yongke Zhang, M.D. "Eque-cel has shown considerable efficacy and safety in clinical trials for refractory autoantibody-mediated neurological diseases in China. We believe it can offer similar benefits for other autoimmune conditions, such as SLE and LN, where autoantibodies are critical pathogenic factors. We are committed to accelerating this innovative therapy to aid more autoimmune disease patients both in China and globally."
So far, Eque-cel has secured three IND approvals from the U.S. FDA for autoimmune indications, including the treatment of Myasthenia Gravis (MG), Multiple Sclerosis (MS), and SLE/LN. Additionally, in China, the product has received two IND approvals from the National Medical Products Administration (NMPA) for Neuromyelitis Optica Spectrum Disorder (NMOSD) and MG. Furthermore, Eque-cel (marketed as FUCASO®) was granted marketing approval by the NMPA in June 2023 for treating relapsed or refractory Multiple Myeloma (R/RMM) in patients who have undergone ≥3 lines of previous therapies including at least one proteasome inhibitor and one immunomodulatory agent.
SLE, a systemic autoimmune disease, is one of the earliest discovered human autoimmune conditions linked to B lymphocyte subpopulation abnormalities. It predominantly manifests with a wide array of organ and system involvements throughout the body, characterized by the presence of numerous autoantibodies. The disease pattern typically alternates between flare-ups and remissions, and without prompt treatment, it can lead to irreversible organ damage and potentially, patient death. According to Frost & Sullivan, there were approximately 8.049 million SLE patients globally in 2023, with about 289,000 in the United States.
Lupus nephritis (LN) is the most severe common complication of SLE, with studies indicating that 60% of SLE patients are likely to develop LN, significantly contributing to the high mortality rate among these patients. Existing standard-of-care treatments for SLE often have limited efficacy, and 14%-33% of LN patients exhibit no response to these treatments."
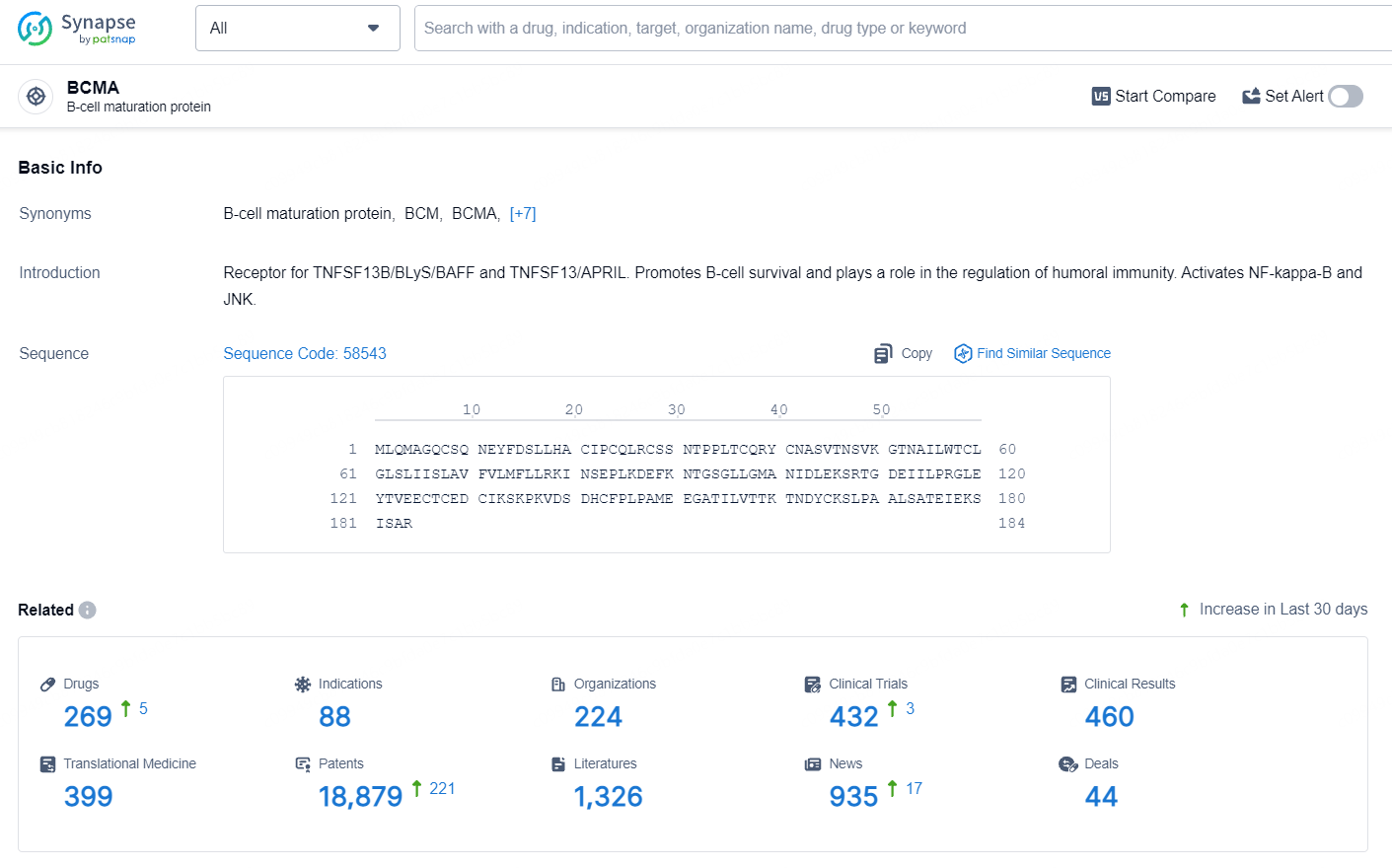
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 12, 2024, there are 16 investigational drugs for the BCMA target, including 31 indications, 29 R&D institutions involved, with related clinical trials reaching 99, and as many as 3210 patents.
Equecabtagene Autoleucel is an individually tailored, BCMA-targeted genetically modified autologous T cell immunotherapy product that identifies and eliminates BCMA-expressing cells. Based on the understanding of the pathological mechanisms of SLE, particularly the pivotal role played by B cells and pathogenic antibodies in the disease, Equecabtagene Autoleucel employs second-generation CAR technology to precisely identify and eliminate cells expressing BCMA. By clearing the activated B cells and plasma cells from the body of the SLE patients, it reduces the pathogenic cells and autoantibodies, thereby suppressing autoimmune inflammation and alleviate the disease.