TG Therapeutics Partner, Precision BioSciences, Gains Approval for Azer-Cel Clinical Trial for Multiple Sclerosis
Precision BioSciences, Inc. (Nasdaq: DTIL), a leading company in gene editing, is using its proprietary ARCUS® platform to innovate in vivo gene editing therapies that address complex genetic modifications, including gene disruption, gene integration, and gene excision. The organization announced that its collaborator, TG Therapeutics, has obtained clearance from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) Application to study Azercabtagene Zapreleucel (azer-cel) in human clinical trials aimed at treating progressive multiple sclerosis. Azer-cel, an experimental allogeneic CAR T therapy discovered by Precision BioSciences and licensed to TG Therapeutics, is targeted at tackling autoimmune diseases. TG Therapeutics plans to initiate a phase 1 clinical trial in 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We extend our congratulations to TG Therapeutics for obtaining IND clearance for azer-cel in patients with progressive multiple sclerosis. Expanding allogeneic CAR T therapies into the realm of autoimmune diseases could potentially pave the way for innovative treatments for those afflicted with chronic conditions," expressed Michael Amoroso, Chief Executive Officer of Precision BioSciences. "We eagerly anticipate TG Therapeutics launching a clinical trial for azer-cel in autoimmune disorders while we advance our proprietary in vivo gene editing portfolio. This includes our intended IND and/or Clinical Trial Application (CTA) submission for PBGENE-HBV targeting hepatitis B within this year."
In January 2024, Precision BioSciences revealed a licensing agreement with TG Therapeutics concerning the CAR T therapy azer-cel. This deal grants Precision worldwide rights to azer-cel for autoimmune diseases and non-cancer indications, bringing upfront and potential near-term economic benefits valued at $17.5 million. Additionally, it includes up to $288 million in milestone payments contingent upon meeting specific clinical, regulatory, and commercial targets, plus high single-digit to low double-digit royalties on net sales.
Precision BioSciences, Inc. is a leading gene editing firm focusing on enhancing life with its innovative ARCUS® genome editing platform. ARCUS is distinct from other gene editing technologies due to its unique cutting mechanism, smaller size, and simplified structure. These unique features may allow ARCUS nucleases to achieve more precise therapeutic outcomes. Precision BioSciences’ pipeline includes in vivo gene editing candidates aimed at providing durable cures for a wide spectrum of genetic and infectious diseases lacking adequate treatments.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
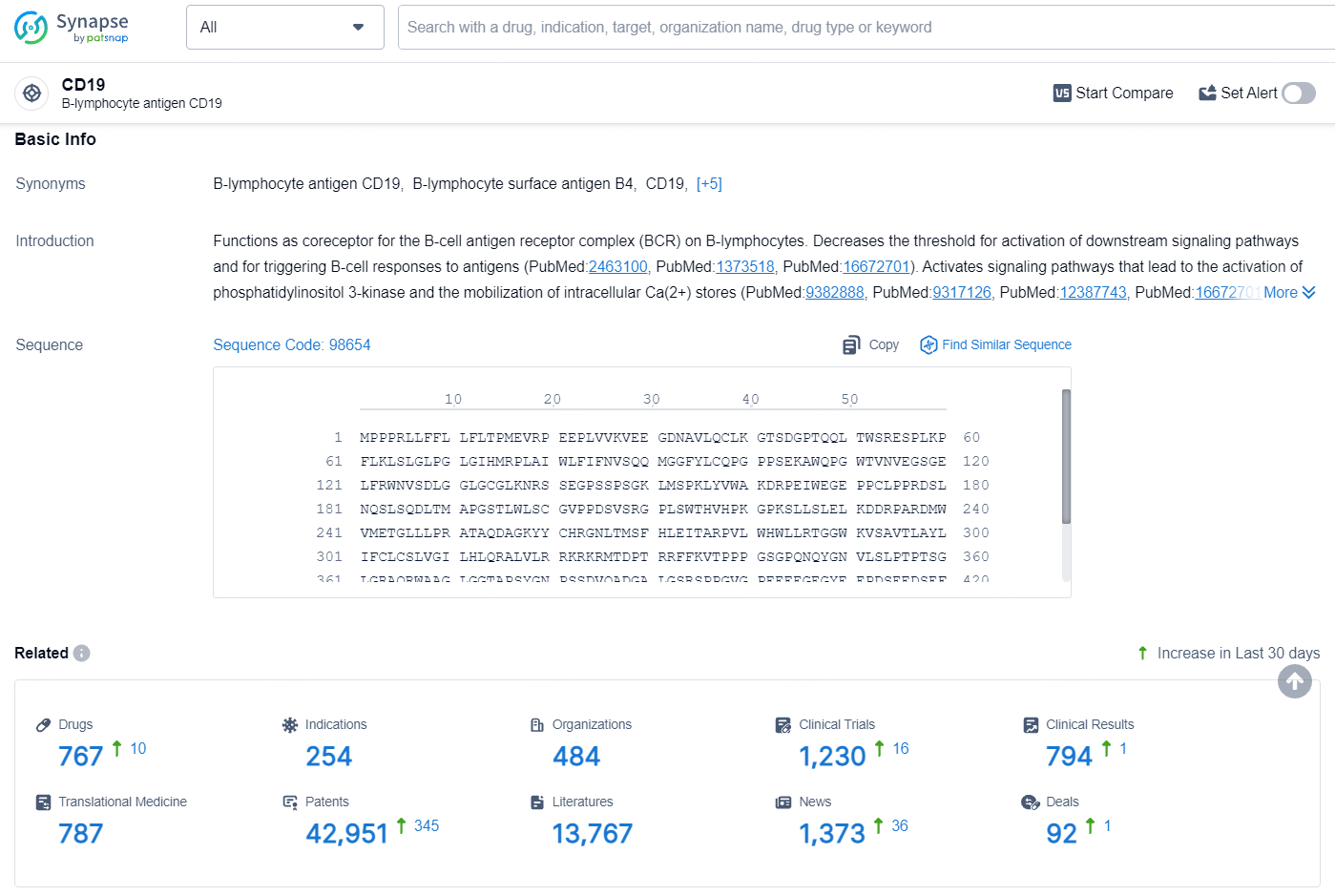
According to the data provided by the Synapse Database, As of August 13, 2024, there are 767 investigational drugs for the CD19 targets, including 254 indications, 484 R&D institutions involved, with related clinical trials reaching 1230, and as many as 42951 patents.
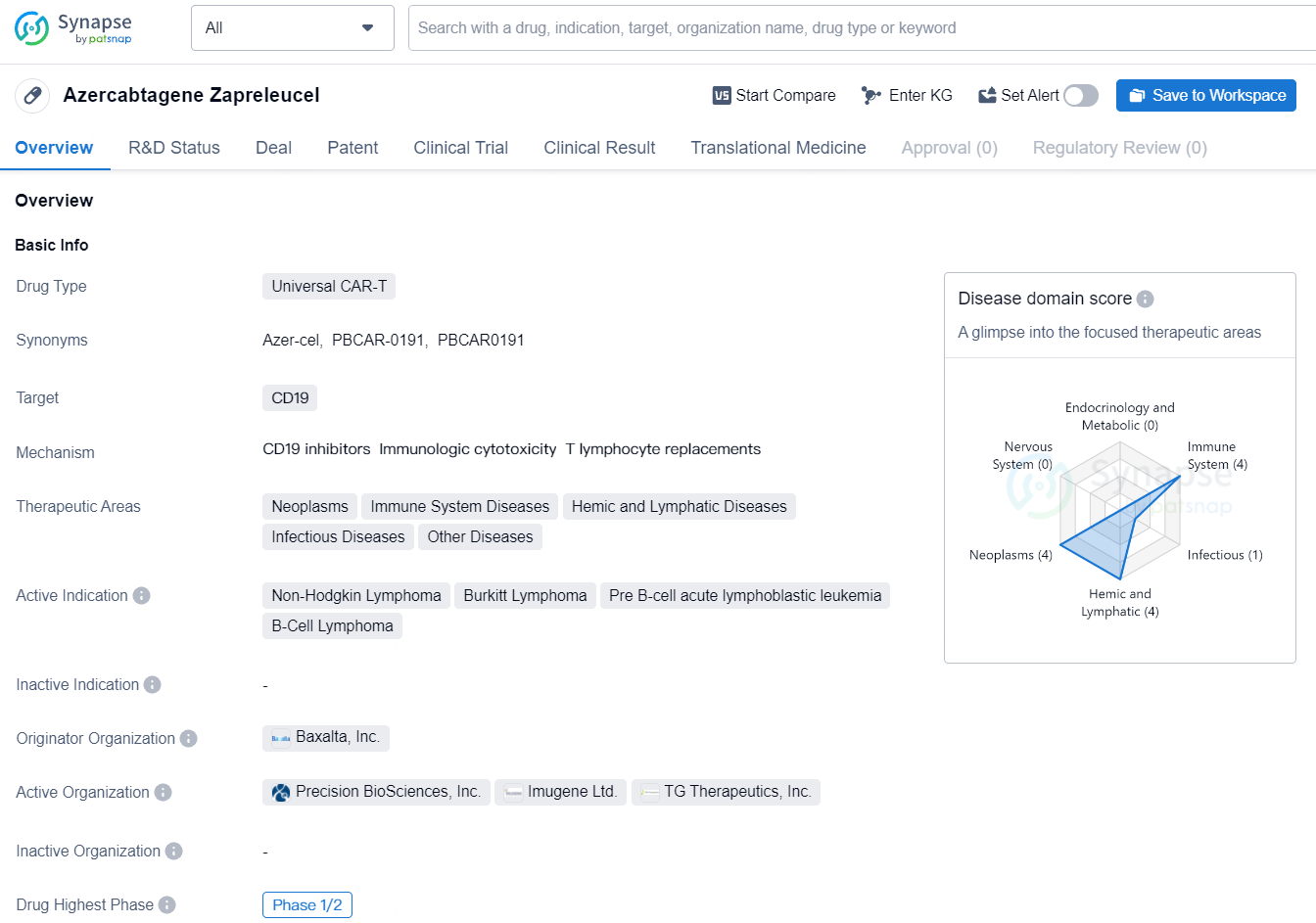
Azercabtagene zapreleucel is a type of Universal CAR-T drug that targets CD19. It is being developed to treat a range of therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, infectious diseases, and other diseases. The drug is currently indicated for non-Hodgkin lymphoma, Burkitt lymphoma, pre B-cell acute lymphoblastic leukemia, and B-cell lymphoma.