FDA authorizes Eylea biosimilar FYB203/AHZANTIVE (aflibercept-mrbb)
Formycon AG, together with their licensing partner Klinge Biopharma GmbH, has disclosed that the FYB203/AHZANTIVE (aflibercept-mrbb), a biosimilar to Eylea, has received approval from the U.S. Food and Drug Administration.
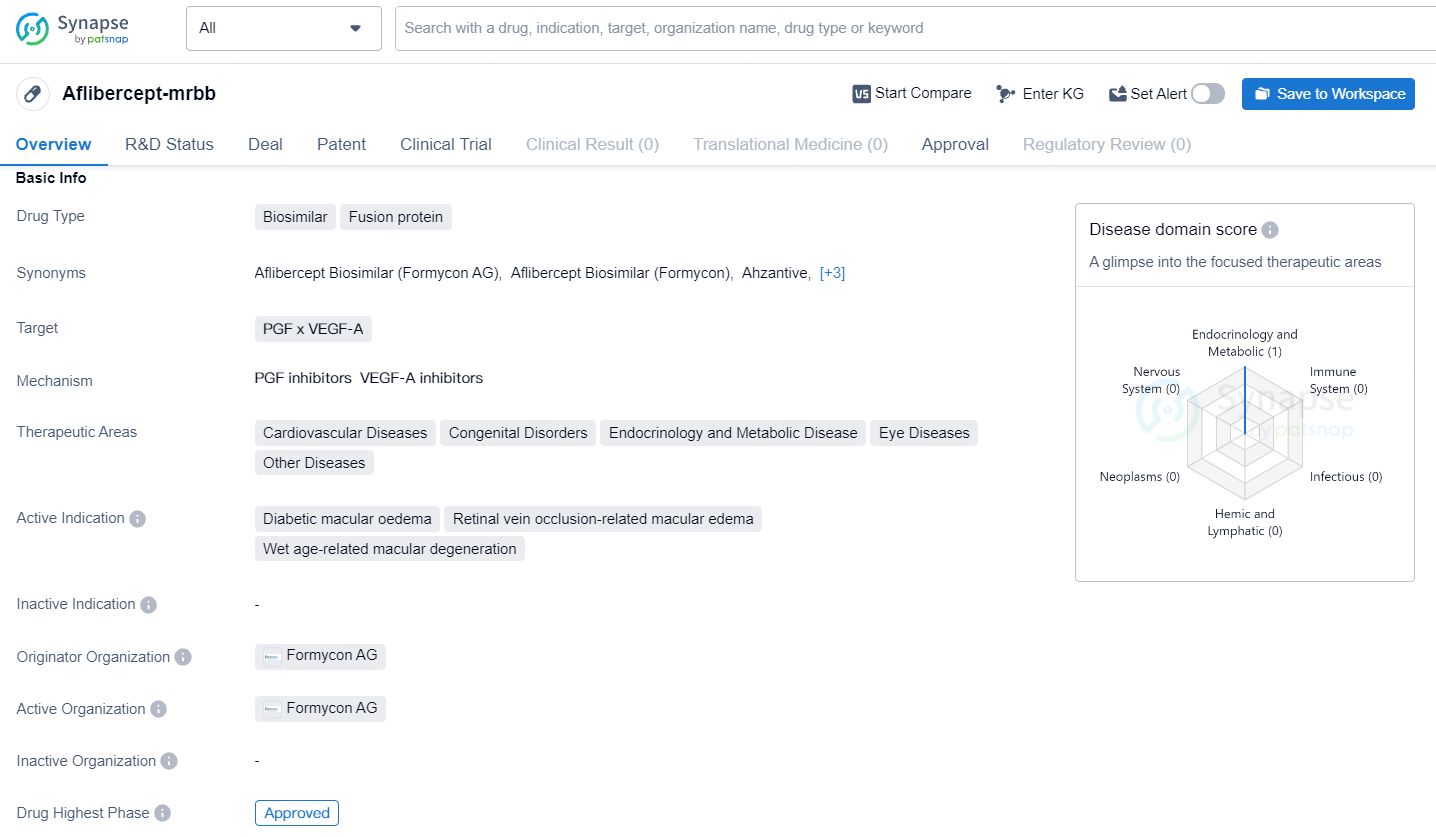
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
FYB203/AHZANTIVE has received FDA approval for treating patients with neovascular Age-Related Macular Degeneration and severe retinal conditions such as Diabetic Macular Edema, Diabetic Retinopathy, and Macular Edema due to Retinal Vein Occlusion. This approval follows an extensive review of comprehensive data, encompassing analytical, pre-clinical, clinical, and manufacturing information. FYB203/AHZANTIVE showed equivalent efficacy, safety, pharmacokinetics, and immunogenicity to the reference medication, Eylea, in patients with neovascular Age-Related Macular Degeneration.
Since the 1980s, biopharmaceuticals have transformed the management of severe illnesses, including cancer, diabetes, rheumatoid arthritis, multiple sclerosis, and ocular diseases. Numerous biotechnology drugs will lose patent protection in the coming years, with medications generating around USD 100 billion in revenue expected to be off-patent by 2025.
Biosimilars are subsequent versions of biopharmaceuticals whose patents have expired. They gain approval through strict regulatory processes in highly regulated markets, ensuring their proven similarity to the original biopharmaceutical reference product. Currently, global biosimilar sales exceed USD 15 billion, with projections suggesting they could surpass USD 74 billion by 2030.
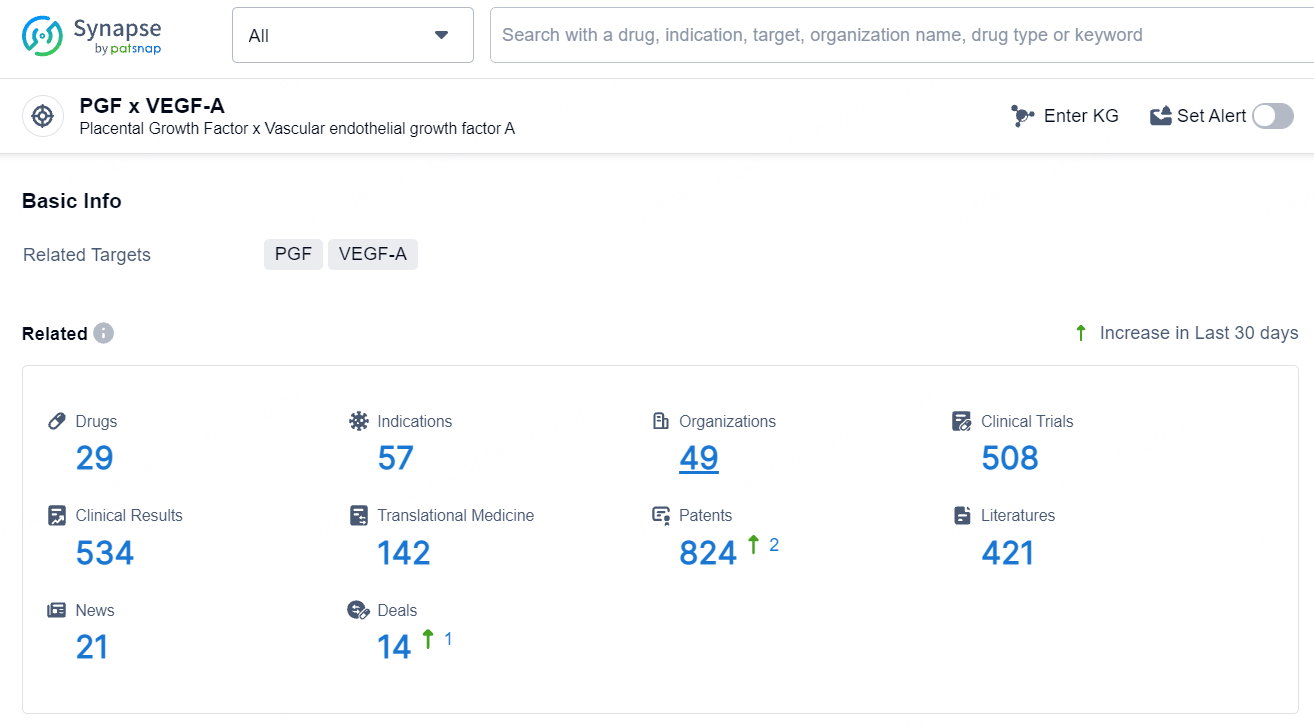
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 5, 2024, there are 29 investigational drugs for the PGF and VEGF-A target, including 57 indications, 49 R&D institutions involved, with related clinical trials reaching 508, and as many as 824 patents.
Aflibercept-mrbb is a biosimilar fusion protein drug that targets PGF x VEGF-A. Aflibercept-mrbb represents a significant advancement in the pharmaceutical industry, particularly in the development of biosimilar drugs for the treatment of various diseases. Its approval and availability in the global market, starting with the United States, provide healthcare professionals and patients with a new treatment option for the specified indications.