Is Tafasitamab approved by the FDA?
Yes, tafasitamab, marketed under the brand name Monjuvi, is FDA approved. Its FDA approval on July 31, 2020, provides hope for patients with limited treatment options, though ongoing studies are necessary to fully understand its long-term efficacy and safety.
What is Tafasitamab?
Tafasitamab is a monoclonal antibody used in combination with lenalidomide to treat adults with diffuse large B-cell lymphoma (DLBCL) who cannot undergo a stem cell transplant. This treatment is specifically used after other cancer therapies have failed or ceased to work.
How Tafasitamab Works
Tafasitamab targets the CD19 antigen on the surface of B-cells, including the cancerous B-cells in DLBCL. By binding to these cells, tafasitamab helps the immune system recognize and destroy them.
Administration and Dosage
Tafasitamab is administered intravenously in a clinical setting by healthcare professionals. The treatment involves a 28-day cycle, with tafasitamab given on specific days in combination with lenalidomide for up to 12 cycles. After this period, tafasitamab is continued as monotherapy until the disease progresses or unacceptable toxicity occurs.
Usual Adult Dose for Lymphoma:
- Cycle 1: 12 mg/kg IV on Days 1, 4, 8, 15, and 22
- Cycle 2: 12 mg/kg IV on Days 1, 8, 15, and 22
- Cycle 3 and beyond: 12 mg/kg IV on Days 1 and 15
Potential Side Effects
Tafasitamab can cause side effects, some of which can be serious. Common side effects include:
- Low blood cell counts
- Fever
- Fatigue
- Cough
- Cold symptoms (stuffy nose, sneezing, sore throat)
- Loss of appetite
- Diarrhea
- Swelling in the hands or lower legs
Serious side effects may include:
- Allergic reactions (hives, difficulty breathing, swelling)
- Infections (fever, cough with mucus, chest tightness)
- Anemia (pale skin, unusual tiredness)
- Low white blood cell counts (mouth sores, skin sores, sore throat)
- Easy bruising or unusual bleeding
Precautions and Warnings
Tafasitamab affects the immune system, increasing the risk of infections, which can be severe or fatal. Patients should be monitored regularly for signs of infection and other potential side effects. Additionally, tafasitamab can harm an unborn baby, so effective birth control is required during treatment and for at least three months after the last dose. Breastfeeding should be avoided during treatment and for three months after the last dose.
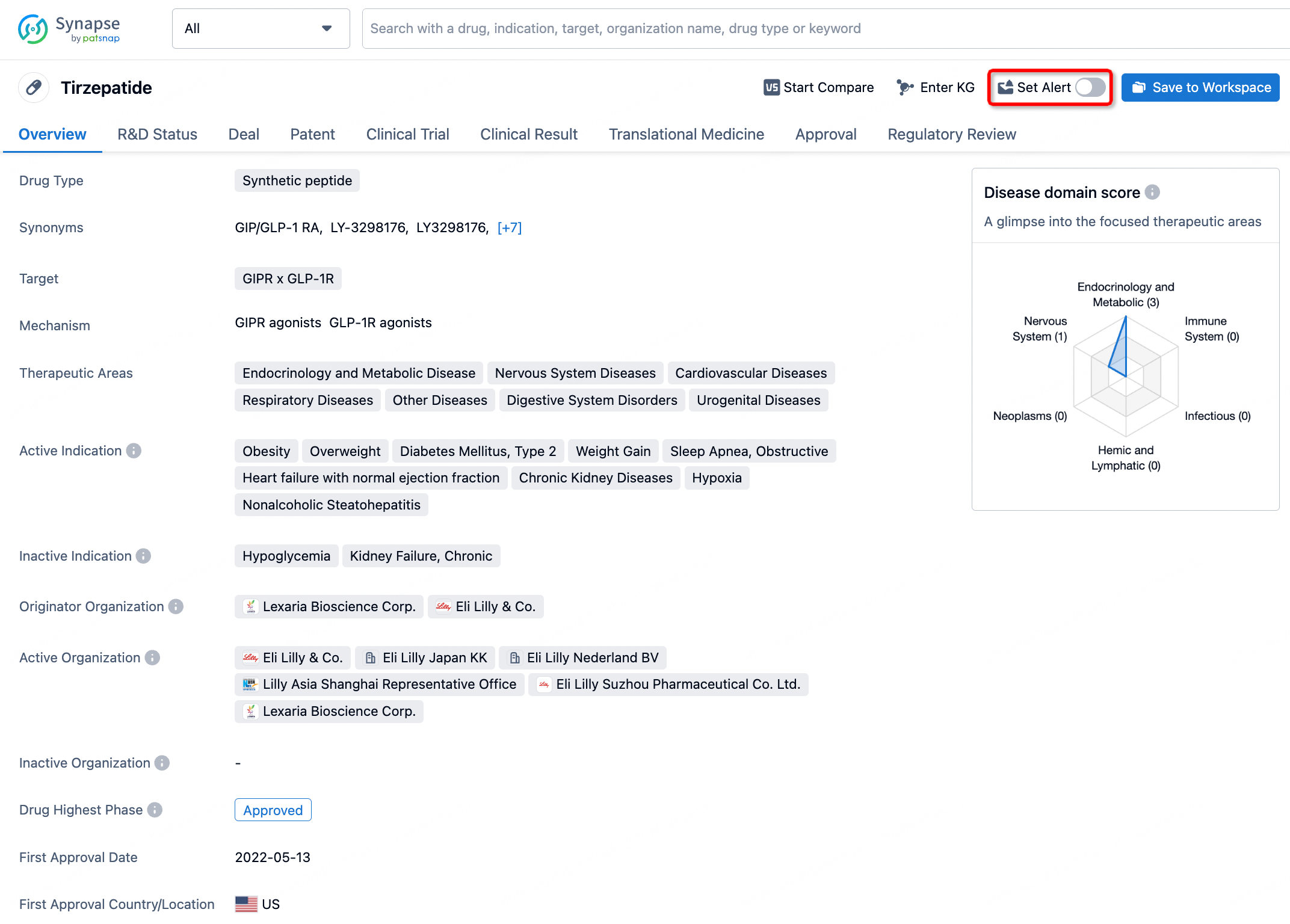
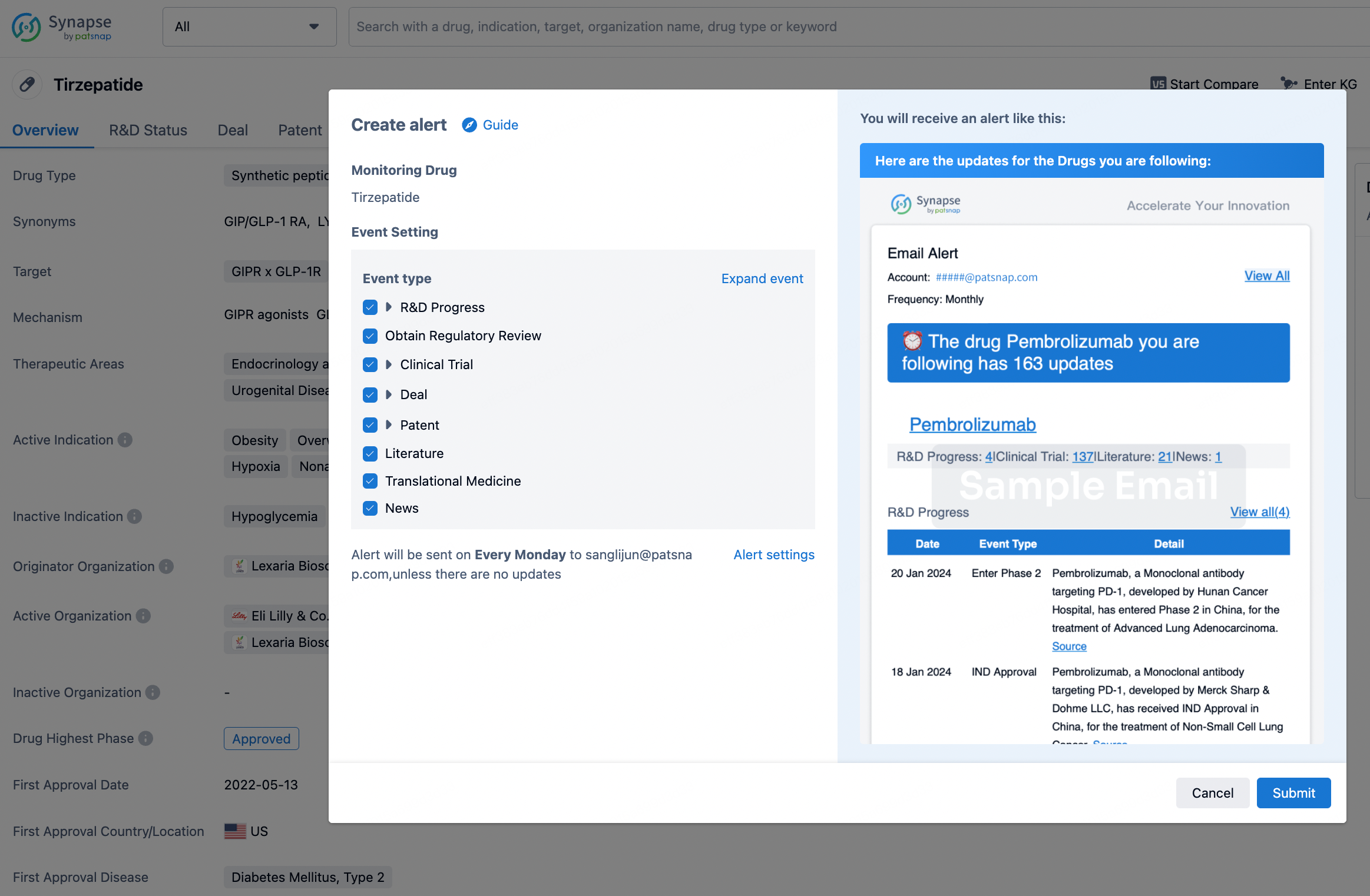
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!