FDA Conditionally Approves Tourmaline Bio's TOUR006 IND Application
BioTechnological Solutions, Inc. (commonly referred to as Tourmaline), a biotech firm in an advanced stage of clinical studies aiming to create groundbreaking treatments to significantly enhance the quality of life for patients dealing with immune disorders that significantly alter their lives, has confirmed that the U.S. Food and Drug Administration (FDA) has given the green light to Tourmaline’s IND application for TOUR006.
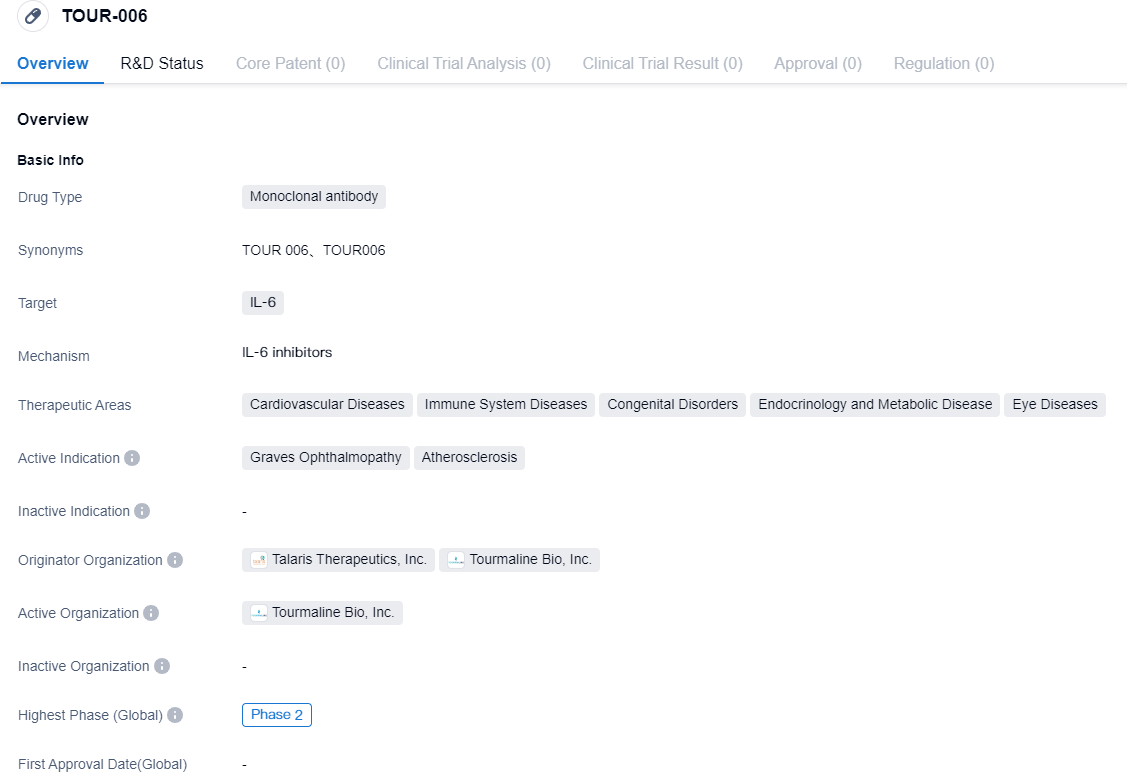
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TOUR006, described as fully-human and demonstrating anti-IL-6 antibodies, shows unique factors such as high level binding affinity for IL-6, and an innately extended half-life period. TED, also referred to as Graves' Orbits, is an immune disorder known for causing inflammation and disfiguration around the ocular region, causing a risk to vision in more serious instances. The unregulated application of IL-6 pathway interrupters in TED has led to a decrease in inflammation and eye protrusion, with an effective impact on crucial biomarkers like pathogenic autoantibody levels.
Sandeep Kulkarni, MD, the CEO of Tourmaline, expressed his excitement over moving forward with TOUR006 into an advanced stage of development in TED. They are of the belief that TOUR006 might be the perfect treatment choice for patients handling TED, due to its anti-inflammatory action, known tolerability capabilities, appealing dose plan, and easy-to-perform subcutaneous administration. "The projection is that the first half of 2025 will yield headline data from this study, with plans to extend TOUR006 development into ASCVD and other potential indications. We are also eagerly awaiting the projected finalization of our merging with Talaris Therapeutics and subsequent listing on Nasdaq in the final quarter of 2023,” said Kulkarni.
The upcoming Phase 2B trial for TOUR006 in TED is set to test 20mg and 50mg doses against a placebo administered by a subcutaneous injection every two months. The expected pool of 81 participants (broken up into three groups of 27 each) will consist of moderate to severe TED patients in an active disease state. The main outcome will be a proptosis response - or a decrease in unusual eye protrusion, taken at the 20-week mark.
About BioTechnological Solutions, Inc.
BioTechnological Solutions, Inc., also known as Tourmaline, is a biotech company in an advanced clinical stage, fueled by its aim to create groundbreaking treatments that can dramatically uplift the quality of life for patients grappling with life-altering immune disorders. The leading initiative by Tourmaline, TOUR006, is an anti-IL-6 antibody displaying unique characteristics such as high binding affinity to IL-6 and a naturally extended half-life period. TOUR006 has already been examined in over 400 patients suffering from autoimmune conditions throughout six clinical studies. The company plans to explore TOUR006 in thyroid eye disease (TED) and ASCVD as its primary two indications, with additional indications under review. In June 2023, Tourmaline revealed that it would merge with Talaris Therapeutics. This combined entity will continue under the name Tourmaline and will be managed by the current Tourmaline team, focused on propelling the development of TOUR006.
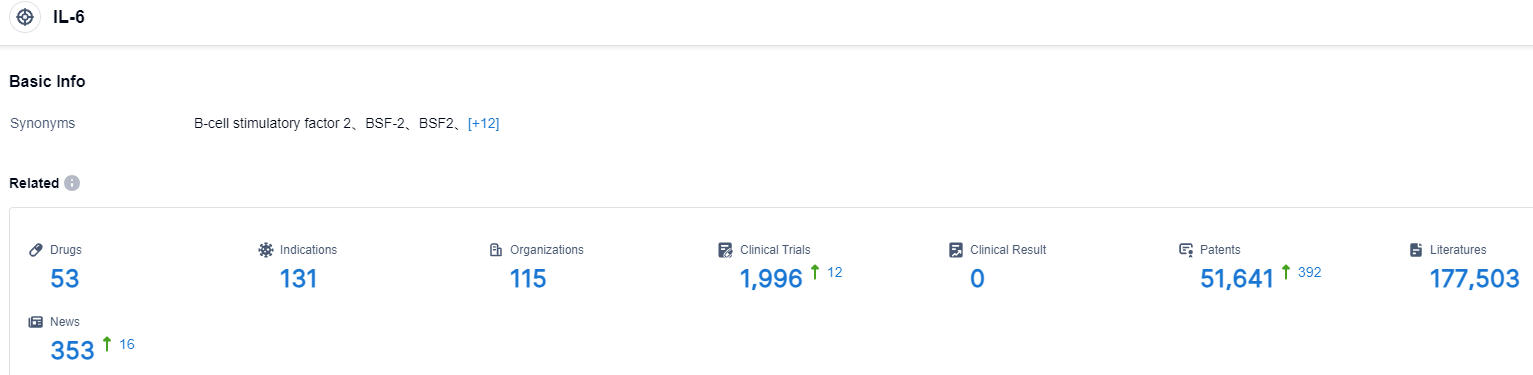
Click on the image below for direct access to the latest R&D progress on IL-6 target drugs, indications, research institutions, clinical trials, and more. As of August 30, 2023, there are 53 investigational drugs for the IL-6 target, including 131 applicable indications, 115 R&D institutions involved, with related clinical trials reaching 1996, and as many as 51641 patents. This Market Spotlight report covers the Thyroid Eye Disease (TED) market, comprising key marketed and pipeline drugs, clinical trials, recent events and analyst opinion, upcoming and regulatory events, probability of success, a 10-year disease incidence forecast, and licensing and acquisition deals, as well as presenting drug-specific revenue forecasts. Data within Market Spotlight reports are sourced from across the Pharma Intelligence suite of products, including Biomedtracker, Trialtrove, and Pharmapremia, as well as Datamonitor Healthcare. The reports also feature content from Scrip, Pink Sheet, Medtech Insight, In Vivo, Generics Bulletin, and HBW Insight.