Pharmaceutical Insights: Colecalciferol's R&D Progress
Colecalciferol's R&D Progress
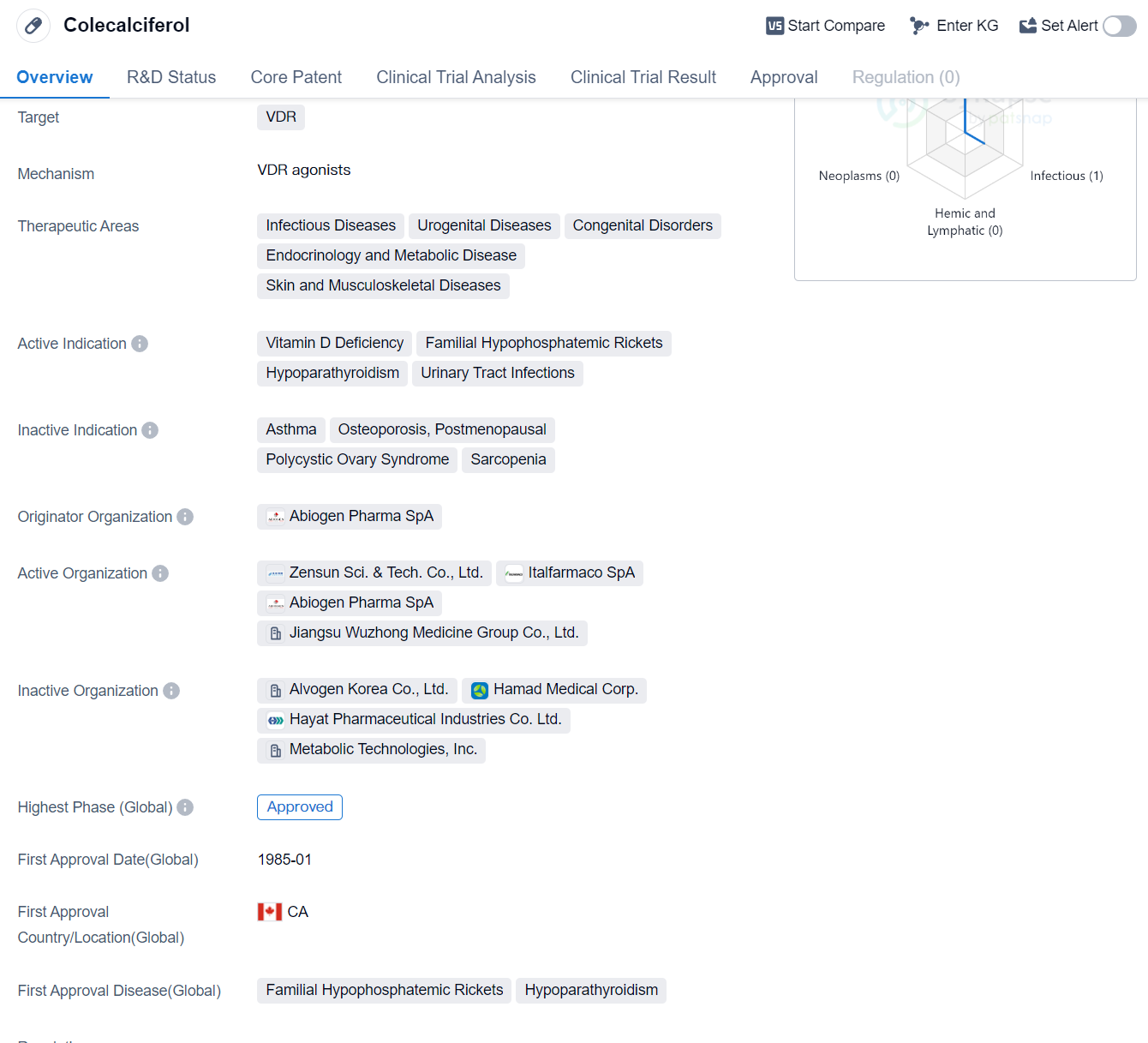
Colecalciferol is a small molecule drug that targets the vitamin D receptor (VDR). It is used in the treatment of various therapeutic areas including infectious diseases, urogenital diseases, congenital disorders, endocrinology and metabolic diseases, as well as skin and musculoskeletal diseases. The drug is indicated for the treatment of vitamin D deficiency, familial hypophosphatemic rickets, hypoparathyroidism, and urinary tract infections.
The originator organization of Colecalciferol is Abiogen Pharma SpA. The drug has reached the highest phase of development which is approved globally. It was first approved in Canada in January 1985.
Colecalciferol is primarily used to address vitamin D deficiency, a condition that can lead to various health problems including weakened bones, muscle weakness, and increased risk of infections. By targeting the VDR, Colecalciferol helps regulate calcium and phosphate levels in the body, promoting healthy bone development and maintenance.
In addition to its role in treating vitamin D deficiency, Colecalciferol is also indicated for familial hypophosphatemic rickets, a rare genetic disorder characterized by low levels of phosphate in the blood. This condition can lead to skeletal abnormalities and impaired growth. By increasing phosphate levels, Colecalciferol helps improve bone mineralization and growth in individuals with this disorder.
Hypoparathyroidism, another indication for Colecalciferol, is a condition where the parathyroid glands do not produce enough parathyroid hormone (PTH), leading to low calcium levels in the blood. Colecalciferol helps increase calcium absorption in the intestines and promotes bone mineralization, helping to restore normal calcium levels in individuals with hypoparathyroidism.
Urinary tract infections, a common condition caused by bacterial invasion of the urinary system, can also be treated with Colecalciferol. The drug's mechanism of action in this indication is not specified in the given information.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Colecalciferol: VDR agonist
VDR agonists are a type of drug that activate the vitamin D receptor (VDR) in the body. The VDR is a nuclear receptor that plays a crucial role in regulating various biological processes, including calcium and phosphate homeostasis, immune function, cell growth, and differentiation.
From a biomedical perspective, VDR agonists are used to treat conditions related to vitamin D deficiency or dysregulation, such as osteoporosis, psoriasis, and certain types of cancer. By activating the VDR, these drugs mimic the effects of vitamin D and help restore normal physiological functions.
VDR agonists can come in different forms, including synthetic compounds or naturally occurring substances that have been modified to enhance their VDR activation properties. These drugs bind to the VDR and trigger a cascade of molecular events that regulate gene expression and signaling pathways associated with vitamin D metabolism.
It's important to note that the use of VDR agonists should be under medical supervision, as they can have side effects and interactions with other medications. Additionally, the dosage and duration of treatment may vary depending on the specific condition being targeted.
Drug Target R&D Trends for Colecalciferol
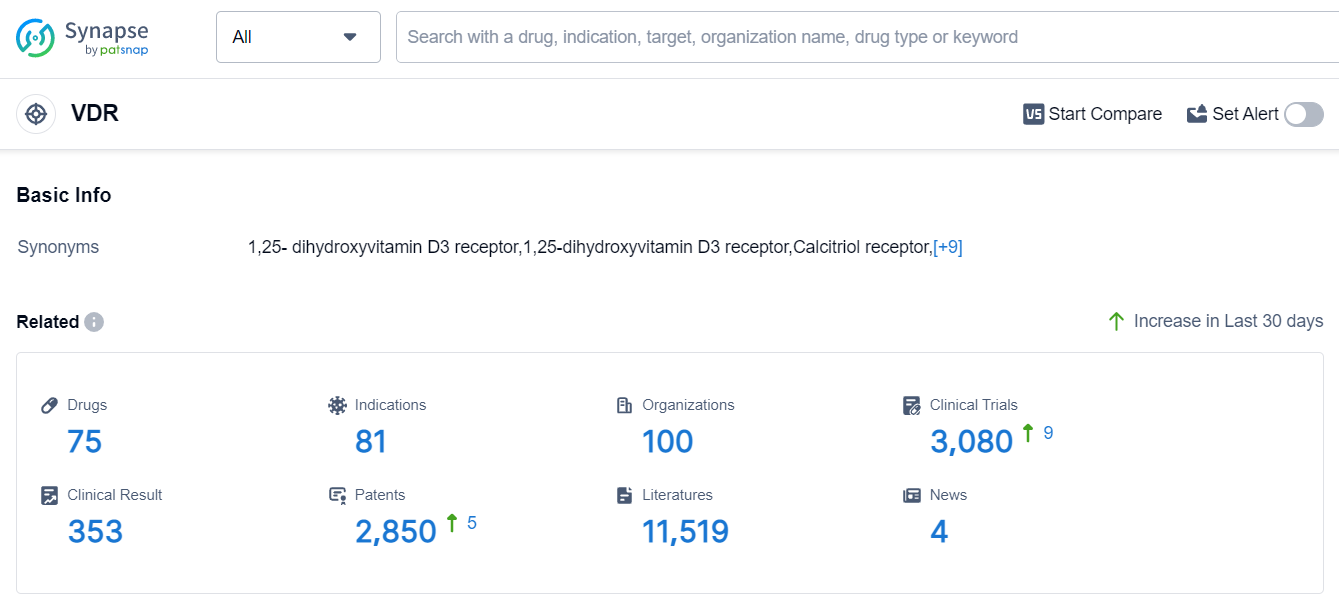
The analysis of the target VDR reveals a competitive landscape with multiple companies actively developing drugs for various indications. Roche Holding AG, LEO Fondet, LEO Pharma A/S, OPKO Health, Inc., and Maruho Co., Ltd. are the companies growing fastest under the target VDR. The indications with the highest number of approved drugs include Hyperparathyroidism, Secondary, Osteoporosis, Psoriasis, and Vitamin D Deficiency. Small molecule drugs are progressing rapidly, indicating intense competition around innovative drugs. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target VDR, with China showing significant progress. Overall, the target VDR presents a competitive landscape with diverse R&D activities and promising future developments.
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 75 VDR drugs worldwide, from 100 organizations, covering 81 indications, and conducting 3080 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Colecalciferol is a small molecule drug that targets the VDR and is used in the treatment of various conditions related to vitamin D deficiency, genetic disorders, and urinary tract infections. Its approval in the global market highlights its potential as a therapeutic option in these areas.