A Comprehensive Review of Daprodustat's R&D Innovations and Drug Target Mechanism
Daprodustat's R&D Progress
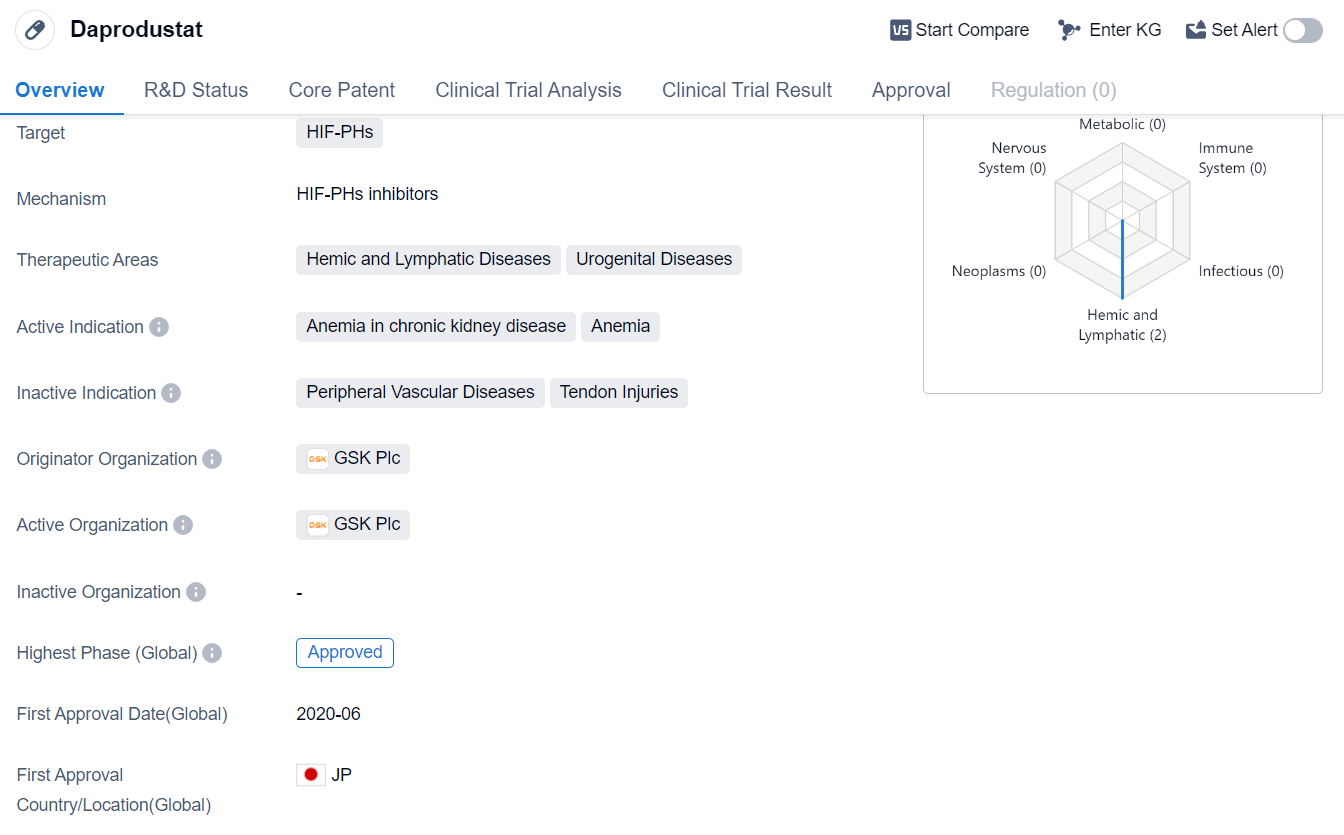
Daprodustat is a small molecule drug that falls under the therapeutic areas of hemic and lymphatic diseases, as well as urogenital diseases. It targets HIF-PHs, which are enzymes involved in the regulation of the hypoxia-inducible factor (HIF) pathway. The drug has been primarily developed to address anemia in chronic kidney disease patients.
The originator organization of Daprodustat is GSK Plc, a renowned pharmaceutical company.
The highest R&D phase of this drug is approved.
The first approval of Daprodustat took place in June 2020, with Japan being the country/location where it was granted. This suggests that the drug has met the necessary safety and efficacy standards set by the Japanese regulatory authorities.
Anemia is a common complication in patients with chronic kidney disease, and it occurs due to the reduced production of erythropoietin, a hormone responsible for stimulating red blood cell production. Daprodustat aims to address this issue by targeting HIF-PHs, which play a role in the regulation of erythropoietin production. By inhibiting these enzymes, Daprodustat can potentially increase erythropoietin levels and stimulate red blood cell production, thereby alleviating anemia in chronic kidney disease patients.
The approval of Daprodustat in Japan signifies a significant milestone in the treatment of anemia associated with chronic kidney disease. It provides healthcare professionals with an additional therapeutic option to manage this condition and improve the quality of life for affected patients. The drug's small molecule nature may offer advantages in terms of ease of administration and potential cost-effectiveness compared to other treatment options.
Further research and development efforts may focus on expanding the indications of Daprodustat beyond anemia in chronic kidney disease. As the drug targets HIF-PHs, which are involved in various physiological processes, there may be potential for exploring its efficacy in other disease areas related to hemic and lymphatic diseases, as well as urogenital diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Daprodustat: HIF-PHs inhibitor
HIF-PHs inhibitors are a type of drug that target and inhibit the activity of hypoxia-inducible factor prolyl hydroxylases (HIF-PHs). HIF-PHs are enzymes that regulate the stability of hypoxia-inducible factor (HIF), which plays a crucial role in the cellular response to low oxygen levels.
From a biomedical perspective, HIF-PHs inhibitors are of particular interest in the field of biomedicine because they have the potential to treat conditions associated with inadequate oxygen supply, such as anemia and ischemic diseases. By inhibiting HIF-PHs, these drugs can stabilize HIF, leading to increased expression of genes involved in oxygen homeostasis, angiogenesis, and erythropoiesis.
HIF-PHs inhibitors have shown promise in preclinical and clinical studies as potential therapeutics for various conditions. For example, in the treatment of anemia, these inhibitors can stimulate the production of red blood cells by enhancing the production of erythropoietin, a hormone that regulates red blood cell production. Additionally, in ischemic diseases, HIF-PHs inhibitors can promote the formation of new blood vessels, improving blood flow to oxygen-deprived tissues.
Overall, HIF-PHs inhibitors represent a novel class of drugs that target the cellular response to low oxygen levels. Their potential applications in treating conditions related to inadequate oxygen supply make them an exciting area of research in biomedicine.
Drug Target R&D Trends for Daprodustat
The analysis of the target HIF-PHs reveals a competitive landscape with multiple companies actively developing drugs in various stages. Akebia Therapeutics, Inc., FibroGen, Inc., and Zydus Lifesciences Ltd. are among the companies with the highest number of drugs in advanced stages. Approved indications primarily focus on anemia-related conditions, while emerging indications like SARS-CoV-2 acute respiratory disease and COVID-19 are also being explored. Small molecule drugs dominate the drug types, indicating intense competition. Japan, China, and the European Union are leading in terms of drug development, with significant progress observed in these regions. Monitoring the progress in China is crucial, as it has shown promising developments. Overall, the target HIF-PHs present a competitive landscape with potential for future growth and innovation in the pharmaceutical industry.
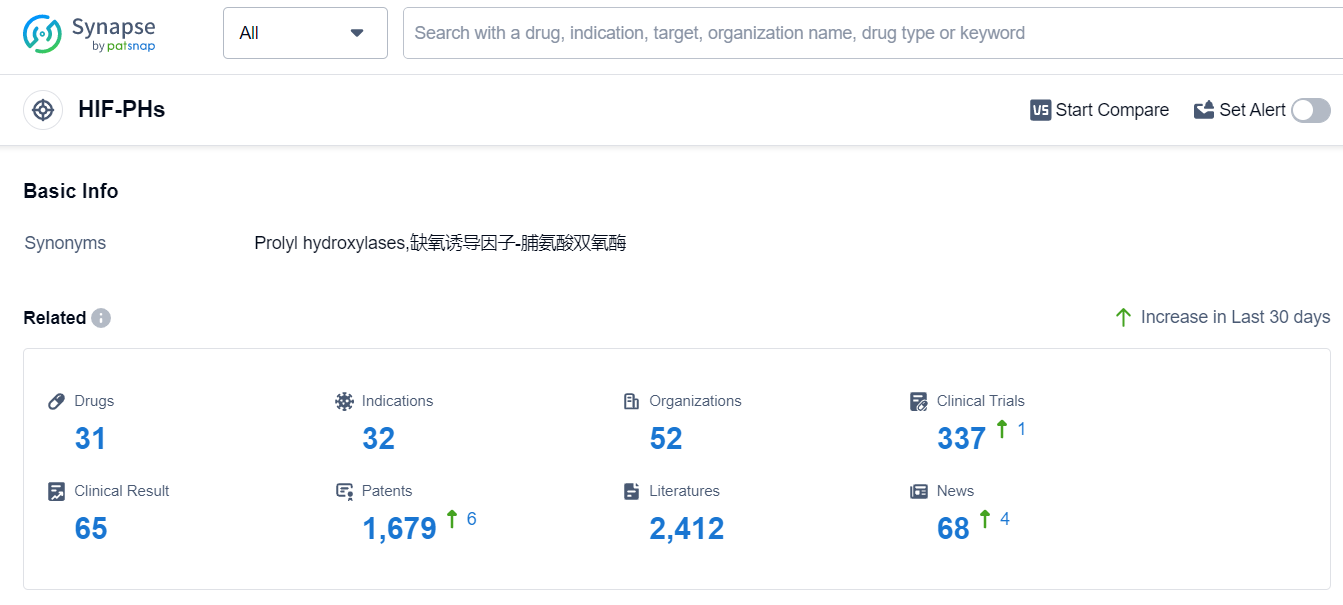
According to Patsnap Synapse, as of 8 Sep 2023, there are a total of 31 HIF-PHs drugs worldwide, from 52 organizations, covering 32 indications, and conducting 337 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Daprodustat is a small molecule drug developed by GSK Plc that targets HIF-PHs. It has received approval for the treatment of anemia in chronic kidney disease patients. The drug's approval in Japan marks a significant advancement in addressing this complication and provides healthcare professionals with an additional therapeutic option. Further research may explore the potential of Daprodustat in other disease areas within its therapeutic scope.