FibroGen's CTGF antibody Pamrevlumab failed in phase III clinical trials for DMD treatment
On August 29, FibroGen announced that the CTGF antibody Pamrevlumab for the treatment of Duchenne Muscular Dystrophy (DMD) in the phase three LELANTOS clinical trial did not achieve its primary NSAA endpoint and crucial secondary endpoints. Consequently, FibroGen's stock price plummeted by 20%.
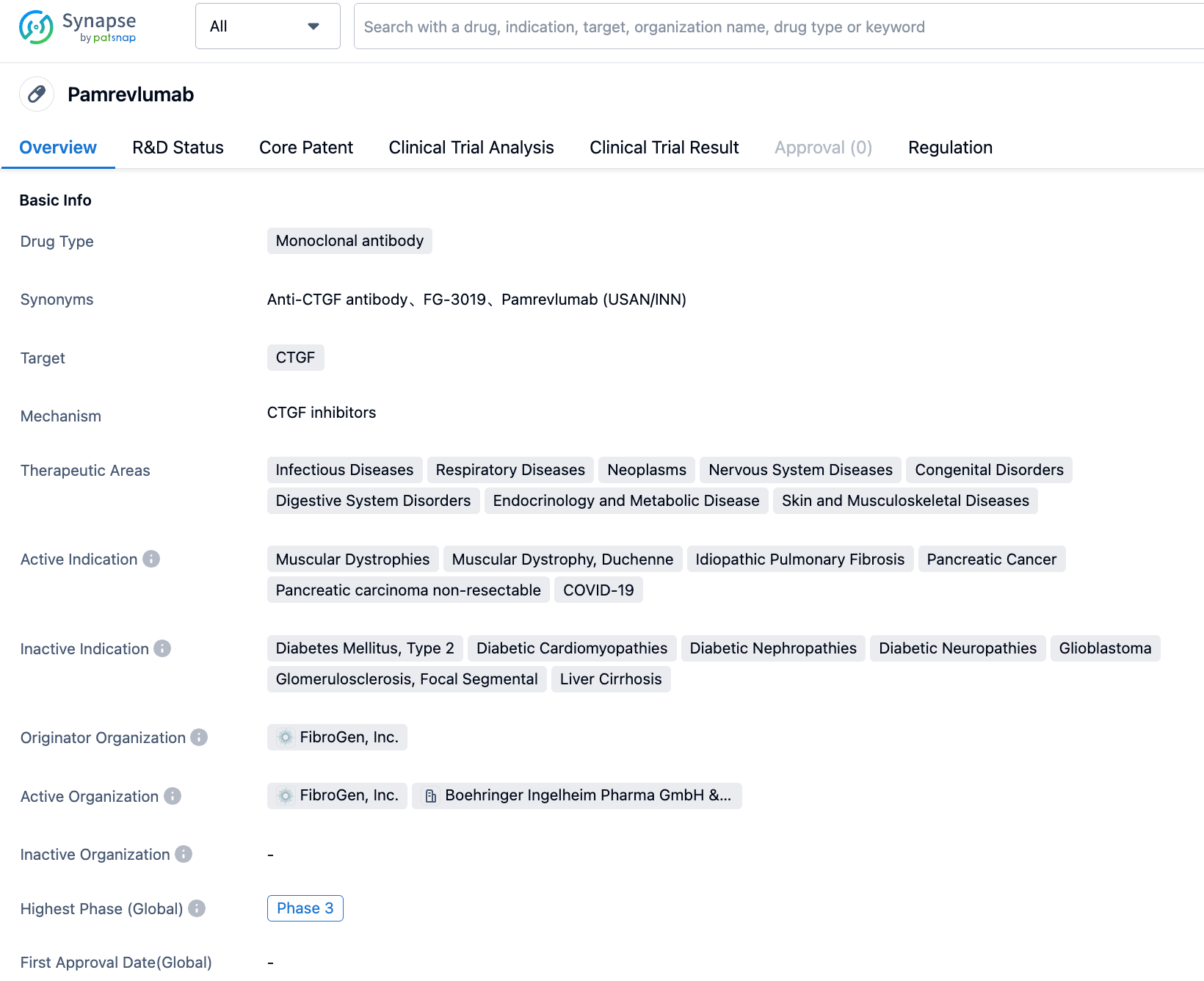
Pamrevlumab is a first-in-class, fully humanized antibody developed by FibroGen that can target and inhibit the activity of CTGF. CTGF is a central mediator of tissue remodeling and fibrosis, involved in a wide range of fibrotic and proliferative diseases that affect the entire organ system. These diseases are characterized by persistent excessive scarring, leading to organ dysfunction and failure, with many of them having virtually no effective treatment options, including Idiopathic Pulmonary Fibrosis (IPF), Pancreatic Cancer, and Duchenne Muscular Dystrophy (DMD). Pamrevlumab has received orphan drug designation for the treatment of IPF, Pancreatic Cancer, and DMD, and has also been granted Fast Track designation by the FDA for the treatment of IPF and locally advanced unresectable pancreatic cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study failed to achieve the primary endpoint of changes in the total NSAA score from baseline to week 52 (placebo-corrected mean difference -0.528 points; 95% CI -2.308 - 1.251; p = 0.5553). The secondary endpoints measured from baseline changes at week 52, the 4-Stair Climb Speed, 10-meter Walk/Run Test, Stand Time, Loss of Ambulation time, and the proportion of patients exceeded 10 seconds in the 10-meter Walk/Run Test, were also not achieved.
On June 26 this year, FibroGen announced the latest results of the phase 3 clinical trial ZEPHYRUS-1 assessing pamrevlumab (the first connective tissue growth factor (CTGF) inhibitor) for the treatment of idiopathic pulmonary fibrosis (IPF). The trial did not achieve the primary endpoint. ZEPHYRUS-1 is a randomized, double-blind, placebo-controlled, multicenter Phase 3 trial designed to evaluate the efficacy and safety of pamrevlumab in participants with idiopathic pulmonary fibrosis (IPF), with a total of 356 participants included. The primary endpoint was the change in forced vital capacity (FVC) compared to baseline at week 48, and the secondary endpoint was disease progression time. Based on the results of ZEPHYRUS-1, FibroGen also regretfully announced it would stop the second phase 3 clinical trial, ZEPHYRUS-2.
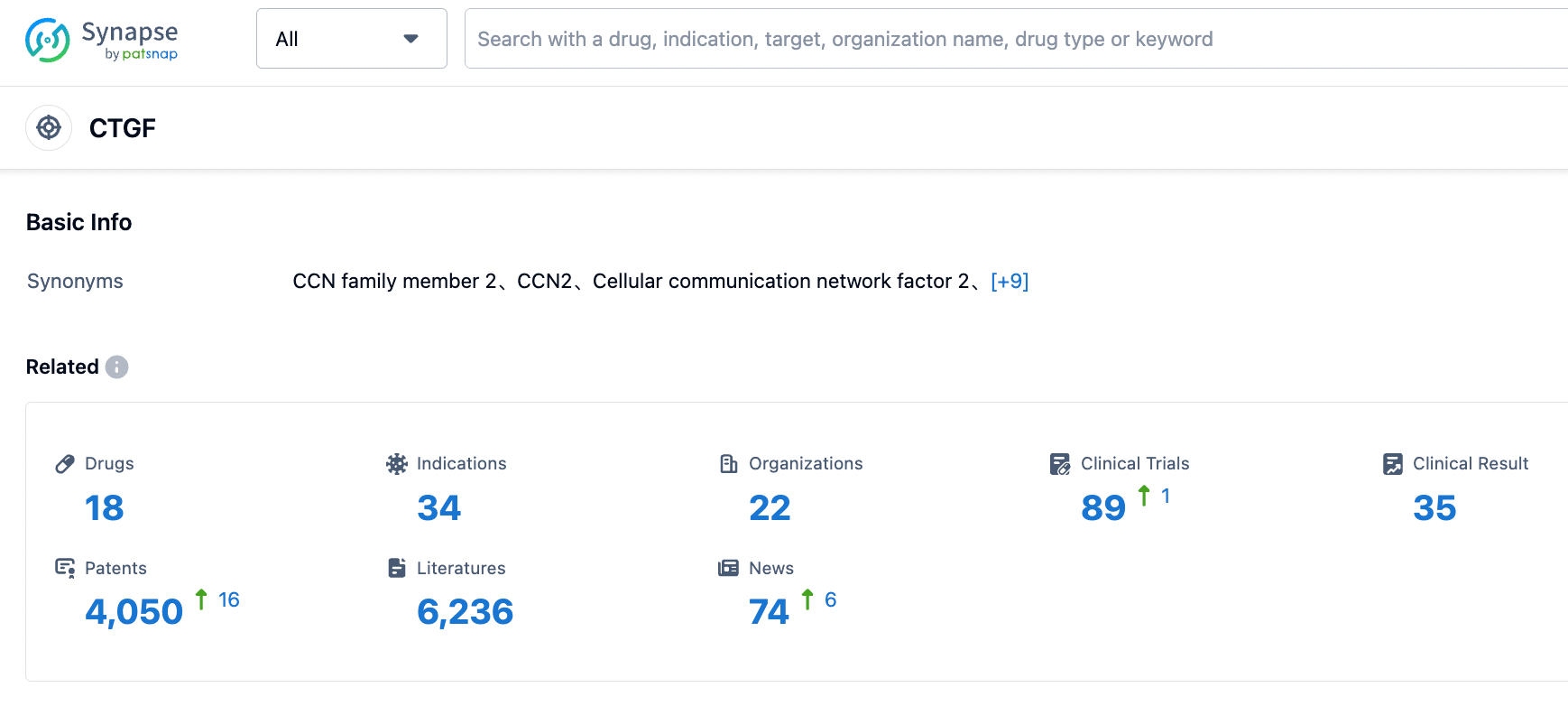
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the information disclosed by the Synapse database, as of August 31, 2023, there are a total of 18 drugs under research targeting CTGF, covering 34 indications, being researched by 22 institutions, involving 89 related clinical trials, and as many as 4051 patents... Currently, FibroGen only has one commercial product, Roxadustat. With the failure of two clinical indications of Pamrevlumab, FibroGen is facing serious product research and development discontinuity issues.