Initial Dose of ISA104 Administered to First Patient with Chronic Hepatitis B in Phase 1 Human Trials
ISA Pharmaceuticals B.V., a clinical-phase biotech firm engaged in creating immune-based treatments for cancer and severe infectious diseases, is delighted to declare the initial administration of the medication to a patient in the HEB-PEP trial. This inaugural human application (FIH) phase 1 examination, is exploring the efficiency of ISA104, which is a breakthrough medical approach aiming to realize a functional cure in patients with Chronic Hepatitis B. The drug administration was conducted at the Hepatology Division of the Erasmus Medical Center in Rotterdam, the supporting entity behind this dosage amplification trial.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Chronic infection of the Hepatitis B virus results in a grave liver disease and global health risk. Should the patient not successfully rid themselves of the virus, they are carriers, with the potential for serious liver conditions including liver cancer, liver failure, and/or cirrhosis to develop. Currently, there are no curative treatments for these conditions. Roughly 2 billion people across the globe have experienced an infection of the Hepatitis B virus. Every year, about 1.5 million new infections occur. Nearly 300 million individuals live with the long-term consequences of this illness.
ISA Pharmaceuticals, in association with Erasmus MC, has utilized Synthetic Long Peptide (SLP®) technology to formulate ISA104 immunotherapy. The aim of ISA104 is to trigger a robust and particular immune response to combat HBV in order to essentially cure those with chronic infections. Previous studies have illustrated the efficacy of SLP immunotherapy in patients who suffer from chronic human papillomavirus type 16 infection.
ISA Pharmaceuticals' Chief Medical Officer Leon Hooftman announced: "We are incredibly pleased to declare that the first participant has been registered with ISA104 in the HEB-PEP clinical trial, which marks an important objective in our plan to innovate an efficient treatment for those with chronic hepatitis B virus infection, an immense global health problem and notable cause of liver cancer. This study will furnish important data on the safety and potential effectiveness of ISA104, as we proceed to enrich our clinical efforts with an emphasis on bettering patient outcomes.”
The study's Principal Investigators from Erasmus MC's Gastroenterology & Hepatology sector, Dr. Sonja Buschow, and Dr. Dave Sprengers mentioned: "We are thrilled by the advancements we are achieving in this first of its kind human trial and are looking forward to seeing the potential influence that therapy based on ISA104 could have on those battling a chronic hepatitis B virus infection. This trial will let us ascertain whether or not the safety risks and immune responses in patients treated with ISA104 correlate to chance occurrences while also broadening our comprehension of this promising new treatment, of immune responses tailored towards HBV and novel HBV biomarkers."
ISA104 is the latest offering from ISA's ongoing program of clinical development, following the trailblazing ISA101b which is currently in late-stage clinical development for treating HPV16-induced cancers.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this Indication.
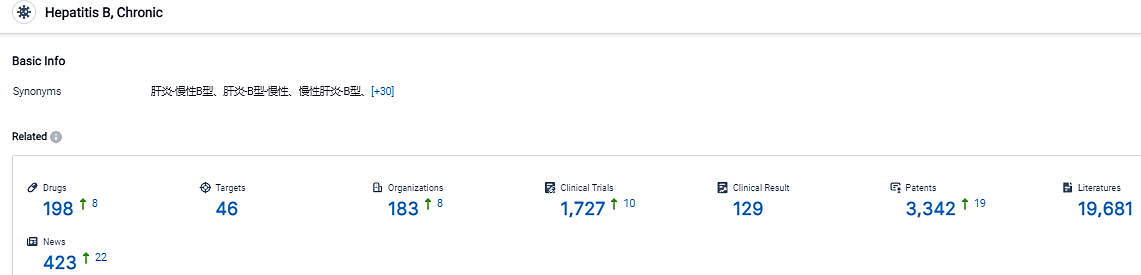
According to the information disclosed by the Synapse Database, as of September 1, 2023, there are 198 investigational drugs for the Chronic Hepatitis B, including 46 targets, 183 R&D institutions involved, with related clinical trials reaching 1727, and as many as 3342 patents. The inflammation and damage to the liver caused by long-term HBV infection increases patients’ risk for developing hepatocellular carcinoma.
Guidelines recommend treatment in patients with HBV DNA levels above 20,000 IU/ml regardless of liver damage, and at lower levels in the presence of signs for liver fibrosis or inflammation. Currently, nucleotide and nucleoside analog antivirals, which directly inhibit viral replication, and immunomodulatory agents are the two approaches used. The ideal aim of antiviral therapy is complete eradication of HBV; however, this aim is currently unattainable as the approved antiviral therapies are unable to overcome the challenge posed by episomal or integrated HBV DNA reservoirs in infected hepatocytes, which are not directly targeted by current antiviral therapies.
Therefore, the primary aim of treatment is “functional cure”, classed as suppression of HBV replication to undetectable levels along with HBsAg loss and formation of anti-HBs antibodies. However, HBsAg loss and seroconversion is only achieved by a very small minority of patients treated with currently available therapies, prompting huge pipeline interest in new modes of action which may improve rates of HBsAg loss when used alongside current therapies.