The Mechanism of Interferons Antiviral Action and Its Biological Activity
Interferon (IFN) is a type of crucial cytokine, capable of directly participating in the antiviral immune response of epithelial cells, mediating the natural immune response to induce the expression of Interferon Stimulated Genes (ISG), hence, swiftly establishing the body's natural immune defense line against viral infections.
Based on the structural characteristics, receptor binding, and biological activity of interferon, human interferons can be divided into three types: Type I, II, and III interferons. Their genes are located on human chromosomes 9p21-22, 12q24.1, and 19q13.13, respectively. Among the three types of human interferons, Type I interferons mainly include IFN-α, IFN-β, and other 20 subtypes, Type II interferons only consist of IFN-γ, and Type III interferons comprise four subtypes including IL-29 (IFN-λ1). Most cells infected by viruses can produce Type I interferon, the secreted IFN-α/β can bind to a common receptor on neighboring uninfected cells, and initiate a paracrine signaling cascade response.
Type I and Type III interferons are considered classical antiviral interferons, with alpha interferons being the main subtype used in clinical antiviral treatment. These mainly include subtypes like alpha-1b, alpha-2b, alpha-2a, etc. Recent studies indicate that Type III interferons have a significant relationship with mucosal immunity, while IFN-gamma also exhibits strong virus inhibitory characteristics.
Regulatory Mechanisms of Interferons
● Classic JAK-STAT signaling pathway
The signaling pathway triggered within the cell after type I and II receptors bind to their respective IFNs is very similar, which is the JAK-STAT signaling pathway.
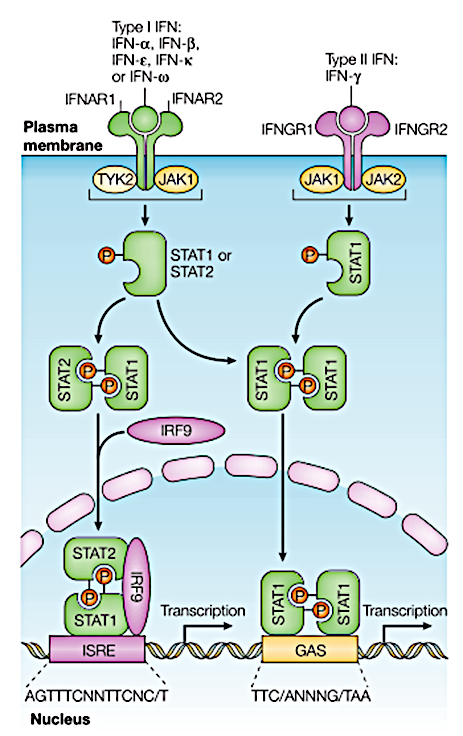
Type I IFN binding to the IFN-α/β receptor can phosphorylate and activate two members of the JAK family: TYK2 (Tyrosine Kinase 2) and JAK1 (Janus Kinase 1). These kinases further phosphorylate STAT1α (STAT91), STAT1β (STAT84), and STAT2 (STAT113). These three activated STATs dissociate from the receptor and bind to the intracellular protein p48, and then this complex enters the cell nucleus and directly binds to the upstream regulatory region (ISREs) of IFN-sensitive genes, thereby regulating the expression of specific genes. Typically, type I and III interferon signals form STAT1-2 heterodimers and ISGF3 complexes by activating TYK2 and JAK1, while type II interferon signals induce the formation of STAT1 homodimers by activating JAK1 and JAK2.
● PI3K/AKT/mTOR signaling pathway
In addition to activating the JAK–STAT signaling pathway, interferons also have an activating effect on the PI3K/AKT/mTOR signaling pathway.
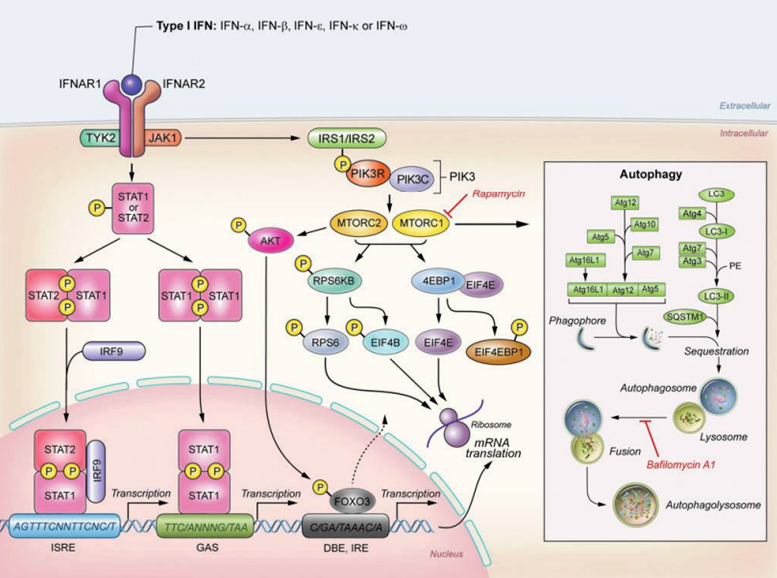
The activation of the PI3K/AKT/mTOR pathway depends on JAK1/TYK2, which phosphorylates IRS1 (insulin receptor substrate 1) and IRS2 (insulin receptor substrate 2). The phosphorylation of IRS1 and IRS2 leads to the activation of PI3K, which subsequently activates the target of mammalian mTORC1 (rapamycin complex 1). MTORC1 activates RPS6kB (ribosomal protein S6 kinase, also known as p70s6 kinase), then phosphorylates RPS6 (ribosomal protein S6), leading to the initiation of mRNA translation. Furthermore, mTORC1 also regulates the phosphorylation of EIF4EBP1 (eukaryotic initiation factor 4E binding protein 1), the phosphorylation of EIF4EBP1 leads to the inactivation of EIF4EBP1, causing the dissociation of EIF4E (eukaryotic translation initiation factor E), thereby initiating the translation of dependent mRNA.
Type I interferons also activate mTORC2 (rapamycin-insensitive complex 2), which is necessary for the phosphorylation of RPS6KB, RPS6, and EIF4BP1.
Biological Activities of Type III Interferons
1) Antiviral Function
Type I interferon is the key antiviral defense and modulation factor in the immune system. On the one hand, it directly activates immune cells; on the other hand, it can indirectly inhibit the replication process of viruses. It can also activate natural killer cells and macrophages, thereby promoting the activation of dendritic cells, while at the same time inducing CD4+ subtype T lymphocytes and T helper cells to produce a large amount of type I interferon on distinct effect cells, protecting CD8+ cells and preventing induced antibody cell death.
2) Antibacterial Function
The antibacterial function of interferons is mainly manifested in IFN-γ. IFN-γ can decrease bacterial iron supply by down-regulating transferrin receptor, or inhibit intracellular bacterial propagation directly by inducing production of endogenous NO. It can also increase the phagocytic body of monocytes and macrophages, lysosome, to dissolve bacteria. By these means, it achieves the function of eliminating bacteria.
3) Anti-parasitic Activity
The anti-parasitic activity of interferon is primarily manifested in IFN-γ. IFN-γ can activate macrophages (Mφ), and the activated Mφ can express high levels of inducible nitric oxide synthase (iNOS) which catalyzes the production of NO from L-arginine. NO has inhibitory and killing effects on inoculated pathogens.
4) Involvement in Immune Regulation
IFN-γ can affect the expression of the Fc receptor for IgG, which facilitates macrophages' phagocytosis of antigens, NK cells' lysis of target cells and the activation of T and B lymphocytes, enhancing the body's immune response. IFN-γ can increase the expression of MHC class II molecules on the surface of macrophages, enhancing their antigen presentation capabilities.
5) Antitumor Activity
IFN-γ is produced by T lymphocytes stimulated by specific antigens. It is the main macrophage-stimulating factor in the body, it resists acid, can activate effector cells, enhances the activity of natural killer cells, macrophages, and tumor-infiltrating lymphocytes, promotes monocyte circulation, increases the expression of antigens and antibodies on the surface of immune cells, stimulates the production of cellular factors such as IL-2, tumor necrosis factor, interferon-α, etc. It inhibits tumor cell division and induces the production of antiviral proteins.