Find Therapeutics Receives FDA Approval for Phase 1 Trial of FTX-101 in Chronic Optic Neuropathy
Find Therapeutics Inc., a biopharmaceutical enterprise dedicated to creating novel treatments for autoimmune disorders, disclosed that the U.S. Food and Drug Administration (“FDA”) has approved its Investigational New Drug (“IND”) application for FTX-101. This approval allows the firm to begin its intended Phase 1 clinical trial of FTX-101 involving healthy participants. The initiation of the study is expected in the fourth quarter of 2024.
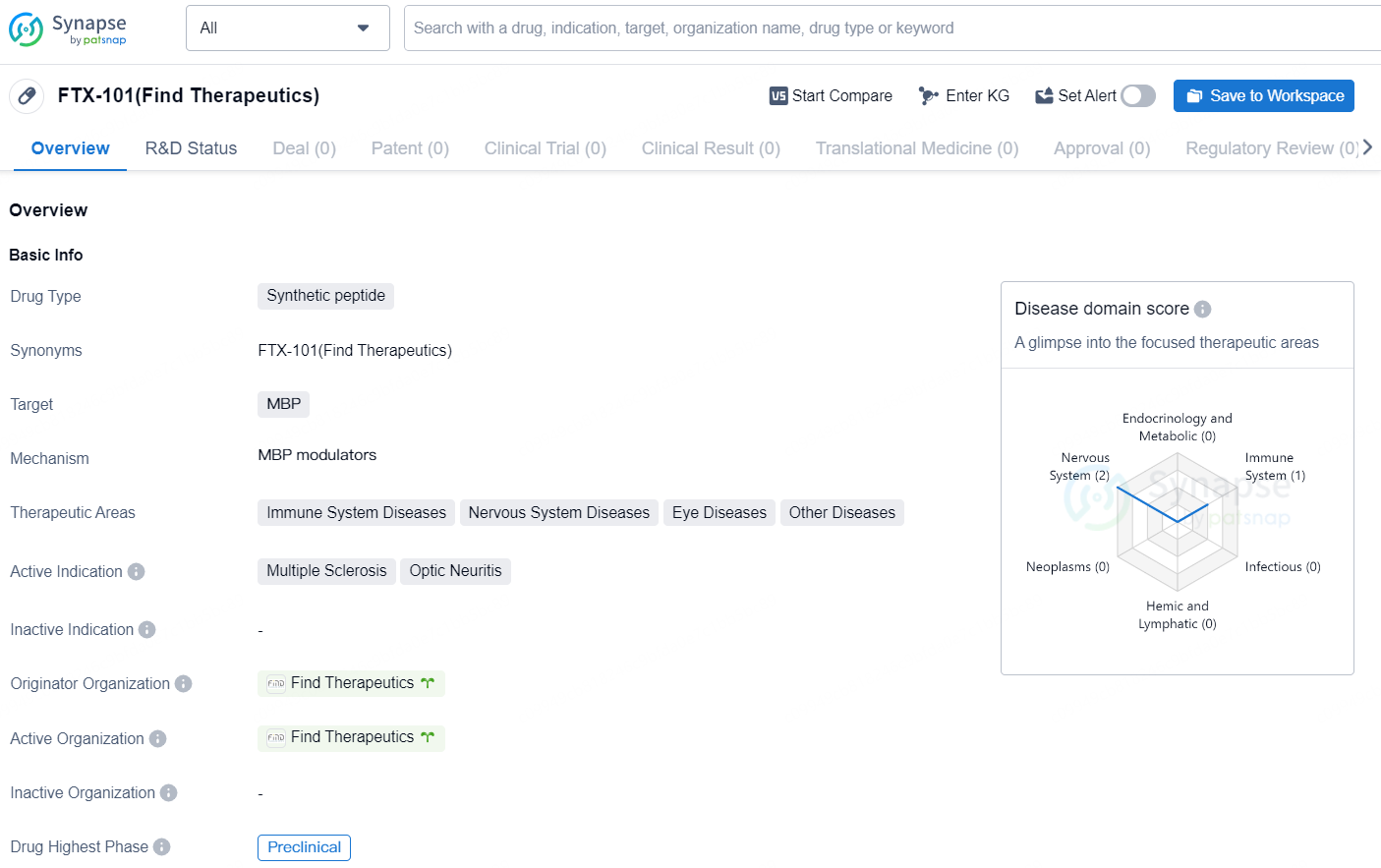
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The FDA’s approval of our IND signifies a critical milestone for Find, enabling us to advance to our Phase 1 trial of FTX-101, a potentially groundbreaking therapy for remyelination currently in development for the treatment of Chronic Optic Neuropathy, or CON," remarked Philippe Douville, CEO of Find. "We are eager to assess FTX-101 in Phase 1 clinical trials, moving us one step nearer to a solution for individuals afflicted by CON, who presently have no approved treatment options available."
The Phase 1 trial aims to be a single ascending dose (SAD) and multiple ascending dose (MAD) study to assess the safety, tolerability, and pharmacokinetics of FTX-101 at varying dose levels. The study plans to include up to 80 participants.
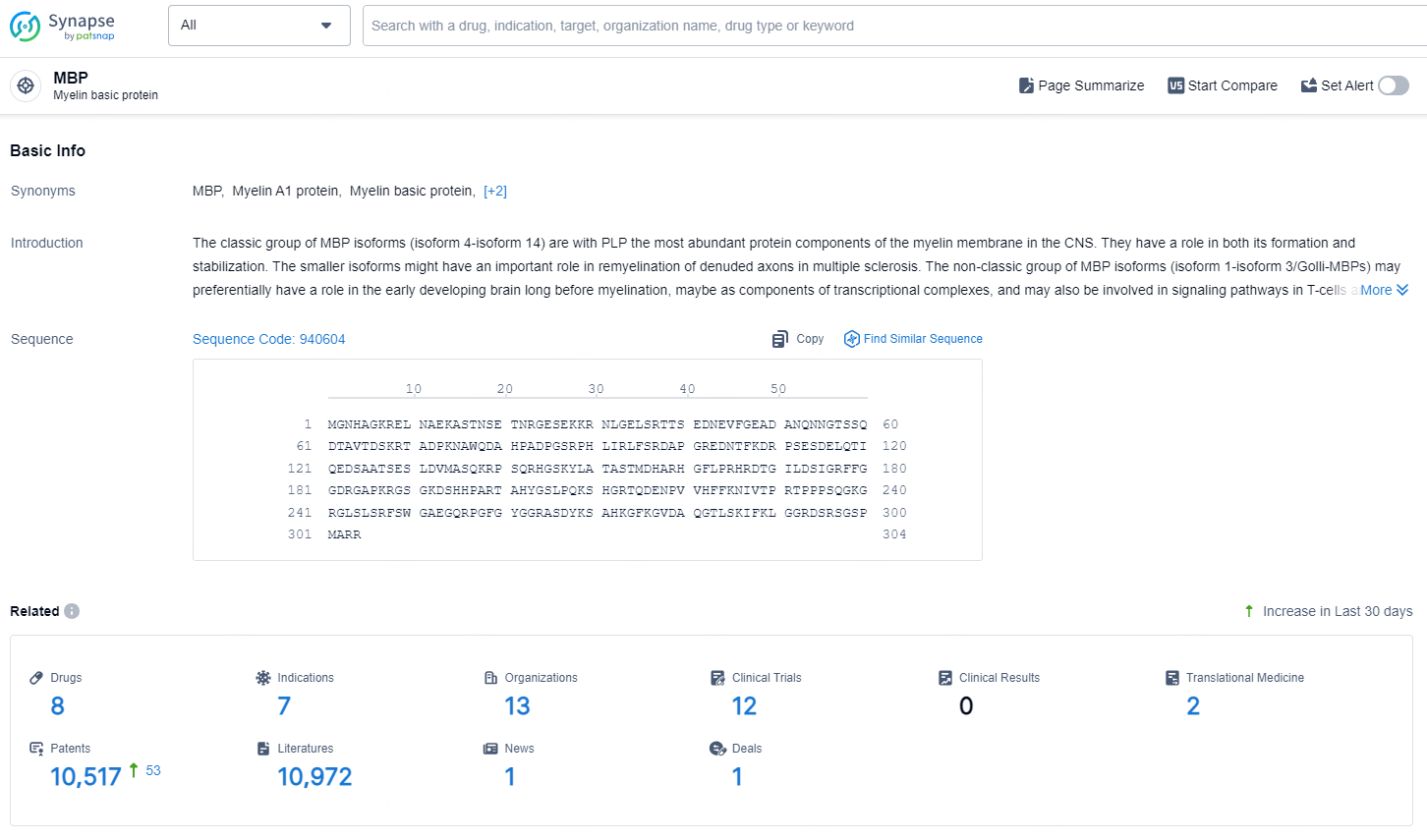
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 30, 2024, there are 8 investigational drugs for the MBP targets, including 7 indications, 13 R&D institutions involved, with related clinical trials reaching 12, and as many as 10517 patents.
FTX-101 is a synthetic peptide drug developed by Find Therapeutics with the primary target of myelin basic protein (MBP). This drug is being developed to address multiple therapeutic areas including immune system diseases, nervous system diseases, eye diseases, and other diseases. The active indications for FTX-101 are multiple sclerosis and optic neuritis. As of the latest available information, FTX-101 is currently in the preclinical phase, which denotes that it is undergoing laboratory and animal testing to assess its safety and efficacy before advancing to human clinical trials. The drug's originator organization, Find Therapeutics, is leading the development of FTX-101.