GT Biopharma Reveals IND Filing for Their New Drug GTB-3650 Targeting CD33+ Leukemia Therapy
GT Biopharma, Inc., an enterprise in the clinical phase specializing in immuno-oncology, is dedicated to creating novel treatments founded on its unique natural killer (NK) cell activator technology, known as the TriKE® platform. The company has recently declared that it has filed an IND (Investigational New Drug) application to the US FDA for its new drug candidate, GTB-3650.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
GTB-3650 is an advanced nanobody-based TriKE® therapeutic agent designed specifically for the management of CD33+ hematologic malignancies, particularly in cases of acute myelogenous leukemia that have not responded to previous treatments and in high-risk cases of myelodysplastic syndrome.
In a statement by Michael Breen, the Executive Chairman and Interim CEO of GT Biopharma, as well as a member of their Board of Directors, he expressed significant enthusiasm about the progress being made with GTB-3650's development. Emphasizing its potential impact for those suffering from CD33+ leukemia, he shared the company's eagerness to introduce this innovative treatment to clinical settings and to achieve their targeted milestones for the year 2024.
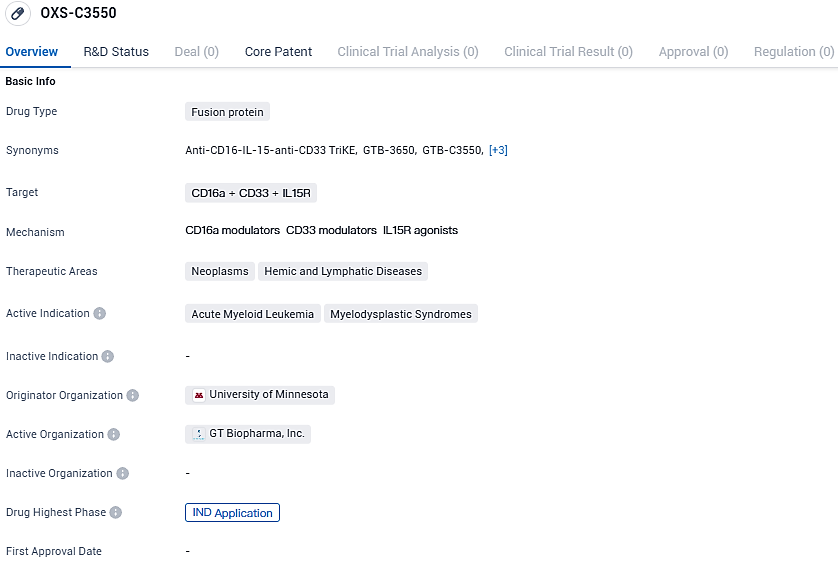
Within the scope of cancer and blood-related conditions, the investigational candidate OXS-C3550 is believed to hold promise for the therapy of a diverse spectrum of oncological and hematologic disorders. Presently under development, it targets acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS), aiming to fulfill the treatment requirements for patients diagnosed with these serious diseases.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
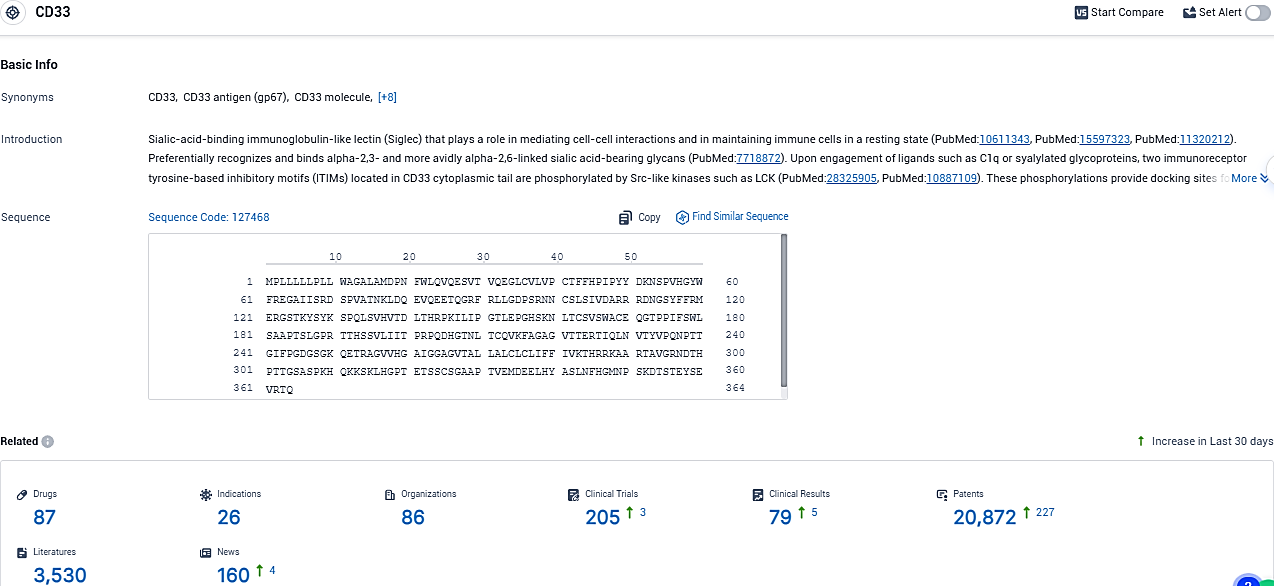
According to the data provided by the Synapse Database, As of December 12, 2023, there are 87 investigational drugs for the CD33 target, including 26 indications, 86 R&D institutions involved, with related clinical trials reaching 205, and as many as 20872 patents.
Overall, OXS-C3550 is intended for the treatment of AML and MDS, and has reached the IND application phase, indicating its progress in the drug development process. Further clinical trials will be necessary to evaluate its safety and efficacy in patients.