Radella Pharmaceuticals Shares Early Results for Groundbreaking Obesity-Fighting Peptide, MD-18
Radella Pharmaceuticals, LLC., an emerging biopharmaceutical firm focused on developing advanced therapies for cardiometabolic conditions, has reported preliminary findings from the Phase 1a MD-18 clinical trial. This study assesses the safety, tolerability, and pharmacokinetics of its experimental MD-18 program aimed at addressing obesity and diabetes. The MD-18 initiative signifies a significant advancement in the inhibition of protein-tyrosine phosphatase 1B (PTP1B), a target proven to be crucial for glucose regulation and energy metabolism. Radella has discovered a series of innovative peptides that specifically target PTP1B by disrupting a precise, non-allosteric interaction with another regulatory protein, effectively mitigating the off-target effects commonly seen with PTP1B inhibitors.
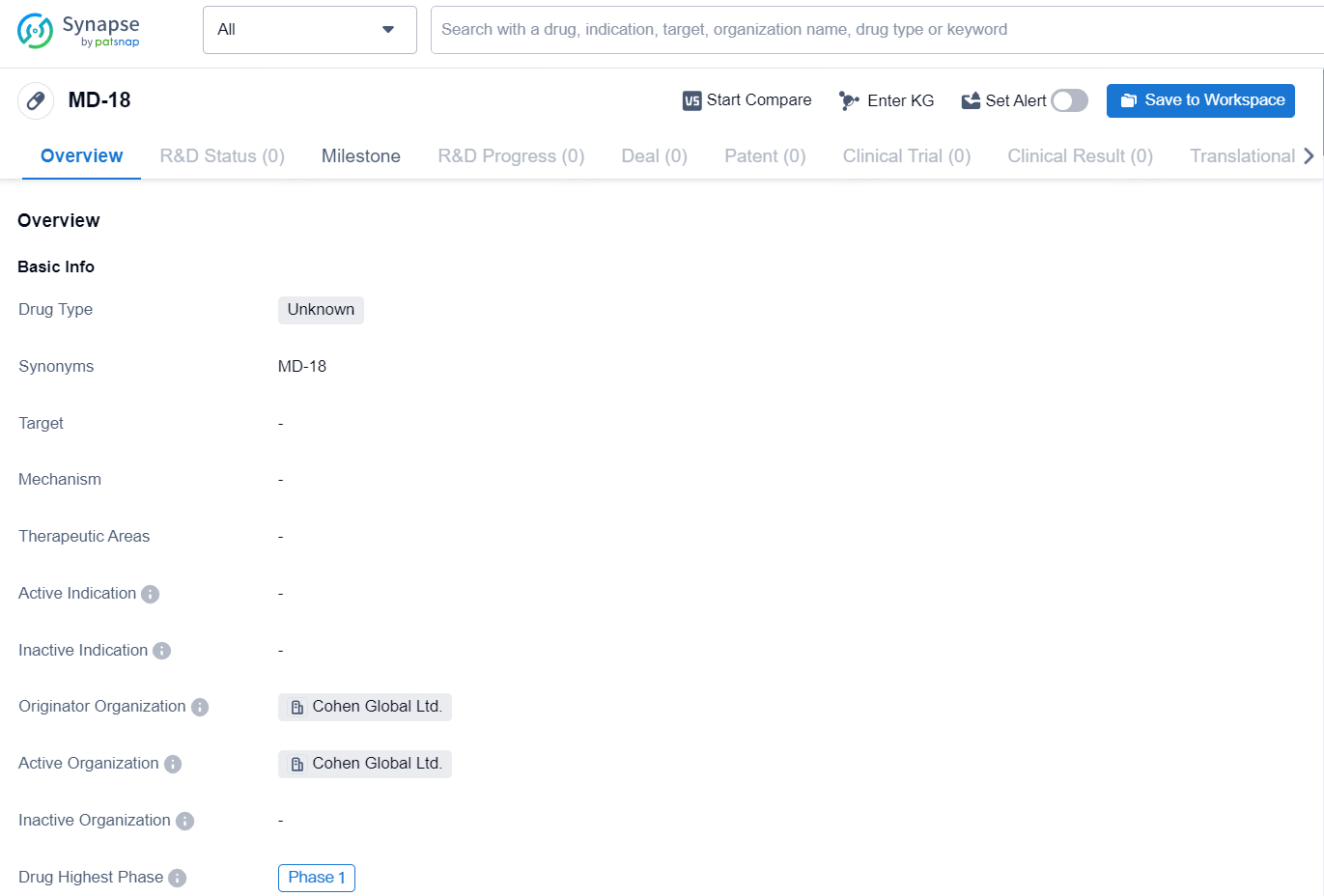
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase 1a trial was a randomized, double-blind, placebo-controlled study conducted at a single center involving 35 healthy participants who received a subcutaneous single dose of MD-18, varying from 40mg to 320mg. Results indicated that MD-18 was safe and well-tolerated at all administered doses, with no serious adverse events or needs for discontinuation. Mild and temporary injection site reactions were the sole treatment-related effects, supporting an encouraging safety profile in humans. Importantly, those treated with MD-18 exhibited significant enhancements in various metabolic parameters after one week, including lowered leptin levels, enhanced insulin sensitivity, and reduced levels of alanine aminotransferase (ALT), LDL-cholesterol, and total cholesterol, none of which were seen in the placebo group. Additionally, subjects reported decreased appetite while on MD-18.
“The outcomes observed after just one dose underscore MD-18's potential to provide significant metabolic benefits,” commented Amir Tirosh, MD, PhD, Chief Medical Officer of Radella, who also serves as Director of the Division of Endocrinology, Diabetes and Metabolism at Sheba Medical Center and is a Professor of Medicine at Tel-Aviv University School of Medicine. “The distinctive mechanism of action of MD-18 addresses both insulin sensitivity and energy expenditure through the regulation of PTP1B and the leptin pathway, offering a potentially transformative solution for individuals with obesity and metabolic disorders.”
MD-18 utilizes an innovative strategy by disrupting a protein-protein interaction (PPI) to modulate PTP1B, which is a well-established therapeutic target. Comprehensive preclinical studies conducted in relevant disease models have shown that the modulation of PTP1B by MD-18 results in considerable improvements in insulin sensitivity and significant weight loss, along with a reduction in adiposity, while maintaining lean muscle mass—features that set it apart from current treatments.
“Present obesity therapies typically concentrate on appetite suppression, neglecting the fundamental causes of metabolic disorders,” noted Daniel Cohen, Founder, Chairman, and CEO of Radella. “MD-18's unique mechanism, which demonstrates promise in enhancing both insulin sensitivity and various important metabolic pathways, has the potential to provide a new treatment option for patients, usable alone or in conjunction with ongoing therapies. Given these promising findings from our Phase 1a study, we are eager to progress our clinical program to thoroughly assess MD-18’s therapeutic capabilities.”
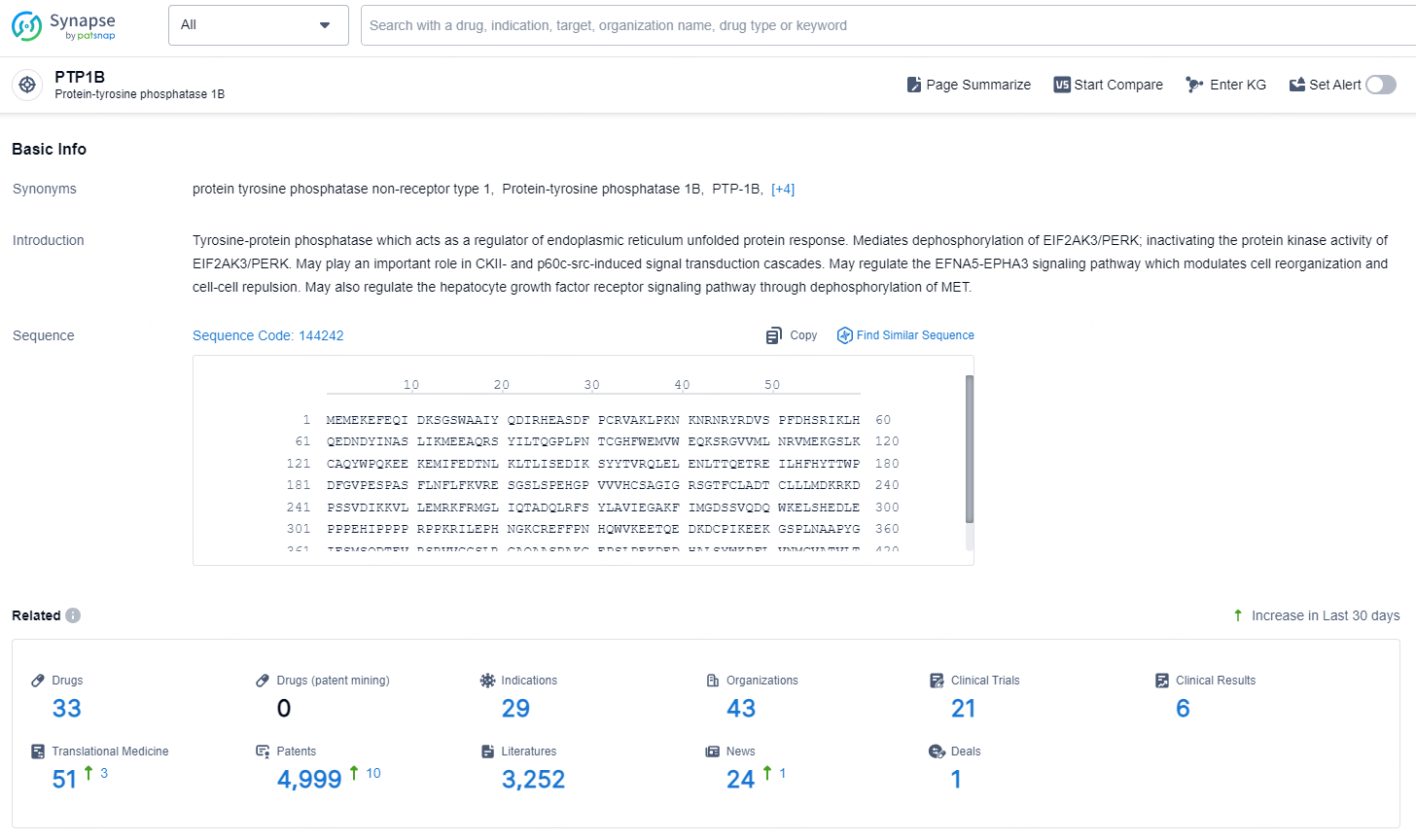
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 11, 2024, there are 33 investigational drugs for the PTP1B target, including 29 indications, 43 R&D institutions involved, with related clinical trials reaching 21, and as many as 4999 patents.
MD-18, into clinical trials, developing a pipeline of follow-on assets targeting unmet needs in other large therapeutic areas, including neurodegenerative and fatty liver disease. MD-18 is a first-in-class peptide that targets multiple pathways involved in obesity and related metabolic conditions. MD-18 demonstrates a breakthrough in targeting protein-tyrosine phosphatase 1B (PTP1B), a historically challenging therapeutic target that plays a crucial role in metabolic regulation. MD-18’s unique mechanism of action, which targets both insulin sensitivity and energy expenditure through PTP1B regulation and the leptin pathway, represents a potentially transformative approach for patients with cardiometabolic disease and its adjacencies.