How to find the sequence of Eplontersen?
Eplontersen, is an antisense oligonucleotide developed by Ionis Pharmaceuticals and Akcea Therapeutics. It targets the transthyretin (TTR) mRNA, which is responsible for the production of the TTR protein. TTR is a transport protein that can misfold and form amyloid deposits, leading to a group of diseases known as transthyretin amyloidosis (ATTR). Eplontersen is primarily indicated for the treatment of hereditary transthyretin-mediated amyloidosis (hATTR) with polyneuropathy, a rare and progressive disease characterized by the accumulation of TTR amyloid in peripheral nerves and other organs, causing significant disability and reduced life expectancy.
Summary of Research Progress of Eplontersen
Eplontersen works by binding to the TTR mRNA, leading to its degradation via RNase H-mediated cleavage. This prevents the synthesis of the TTR protein, thereby reducing the levels of circulating TTR and slowing the progression of ATTR. By targeting the mRNA, Eplontersen aims to address the root cause of the disease, rather than just managing symptoms. Eplontersen is administered subcutaneously, which offers a convenient and less invasive route of administration compared to intravenous infusions. The drug has shown promising results in early clinical trials and is currently in late-stage development. It has received orphan drug designation from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Globally, the competition in the ATTR market is intense, with several companies developing novel therapies. Eplontersen faces competition from other RNA-targeted therapies, such as patisiran (Onpattro) and inotersen (Tegsedi), both of which are already approved for the treatment of hATTR with polyneuropathy. Patisiran is an intravenous RNAi therapeutic, while inotersen is a subcutaneous antisense oligonucleotide. Additionally, there are other emerging therapies, such as vutrisiran (an RNAi therapeutic) and ALN-TTRsc02 (another RNAi therapeutic), which are in various stages of development. Despite this competition, Eplontersen offers a unique advantage with its subcutaneous administration, which may improve patient convenience and compliance compared to intravenous or more frequent subcutaneous injections.
Clinical research on Eplontersen has demonstrated its potential efficacy and safety. The Phase 3 NEURO-TTRansform trial, which is ongoing, is evaluating the safety and efficacy of Eplontersen in patients with hATTR with polyneuropathy. Preliminary data from earlier trials have shown that Eplontersen significantly reduces serum TTR levels and improves neurological function in treated patients. The drug was generally well-tolerated, with common side effects including injection site reactions and mild to moderate gastrointestinal symptoms. Ongoing and future trials aim to further evaluate the long-term safety and efficacy of Eplontersen, as well as explore its potential in other forms of ATTR, such as cardiomyopathy.
Sequence Characteristics of Eplontersen
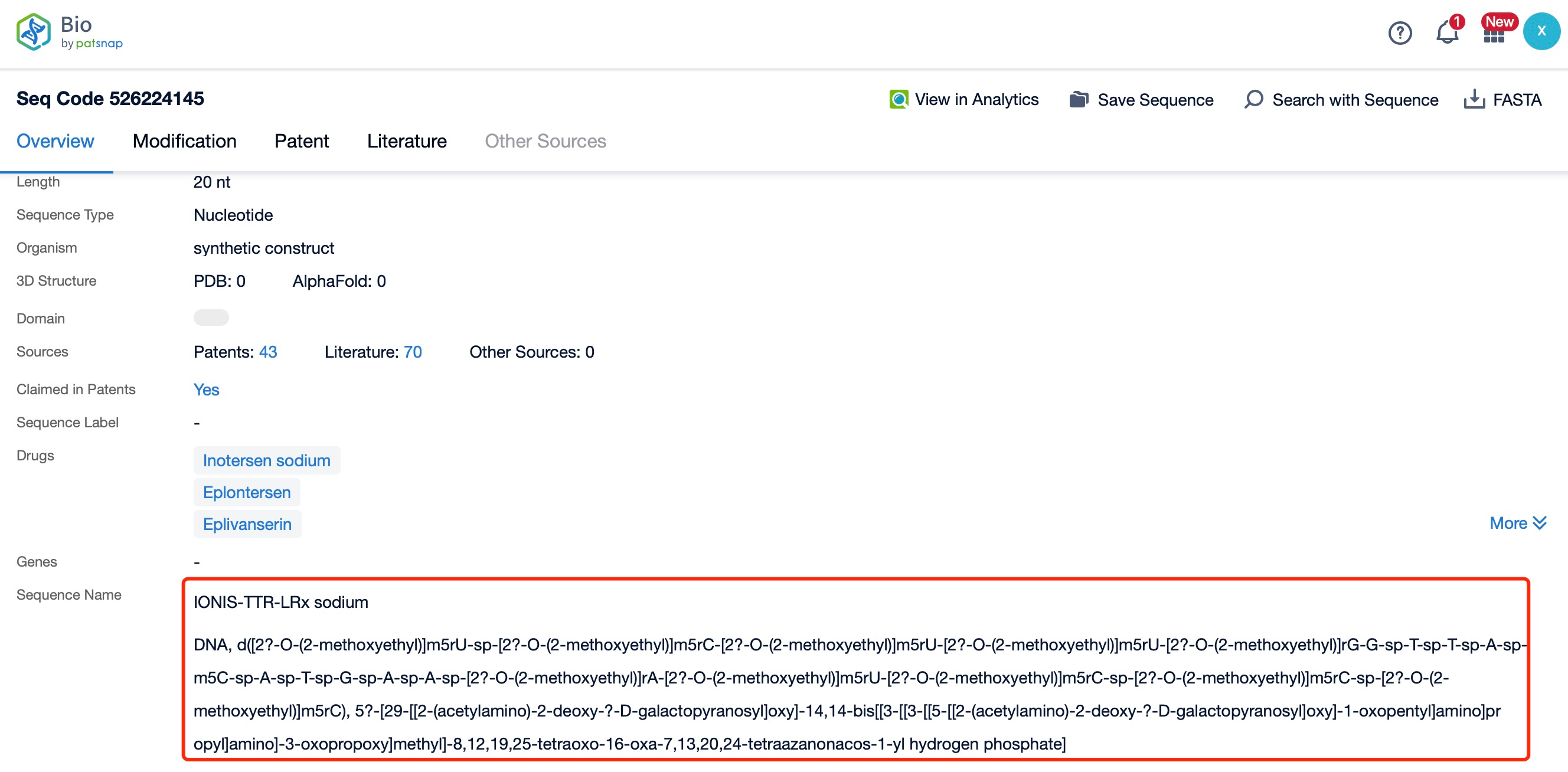
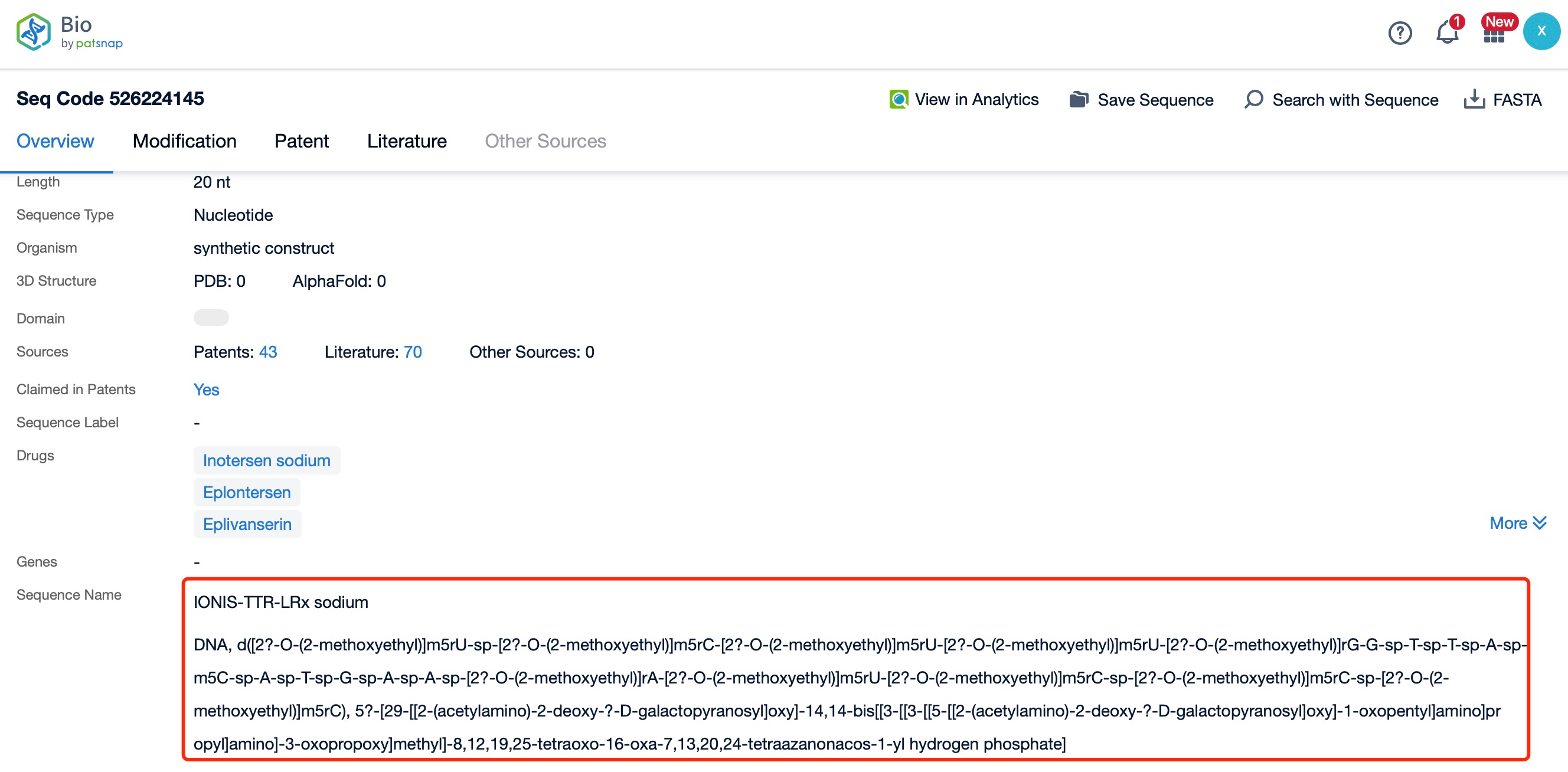
Eplontersen is a 20-nucleotide phosphorothioate-modified oligonucleotide. The sequence of Eplontersen is specifically designed to bind to the TTR mRNA, ensuring high specificity and efficiency in targeting the mRNA. The sequence is optimized to maximize binding affinity and minimize off-target effects, which is crucial for the drug's therapeutic efficacy and safety. The precise sequence of Eplontersen is a proprietary information, but it is known to be highly complementary to the target TTR mRNA, allowing for effective RNase H-mediated cleavage and degradation.
Chemical Modification and Species of Eplontersen
The chemical modifications in Eplontersen include phosphorothioate linkages and 2'-O-methoxyethyl (2'-MOE) modifications. The phosphorothioate backbone replaces one of the non-bridging oxygen atoms in the phosphate group with a sulfur atom, enhancing the stability of the oligonucleotide and protecting it from nuclease degradation. The 2'-MOE modifications are introduced at specific positions along the oligonucleotide to further increase its stability and binding affinity to the target mRNA. These modifications also improve the pharmacokinetic properties of the drug, such as its half-life and tissue distribution.
The phosphorothioate modifications in Eplontersen provide several key advantages. First, they significantly enhance the stability of the oligonucleotide, allowing it to remain active in the cellular environment for a longer period. This increased stability is crucial for the drug's effectiveness in binding to and degrading the TTR mRNA. Second, the phosphorothioate backbone improves the pharmacokinetic profile of the drug, increasing its half-life and bioavailability. This means that the drug can be administered less frequently, which is beneficial for patient convenience and compliance. Third, the phosphorothioate modifications reduce the risk of off-target effects by enhancing the specificity of the oligonucleotide for its target mRNA.
The 2'-MOE modifications in Eplontersen also play a critical role in the drug's performance. These modifications increase the binding affinity of the oligonucleotide to the target mRNA, ensuring efficient and specific inhibition of TTR protein synthesis. The 2'-MOE groups are hydrophobic, which helps to stabilize the oligonucleotide in the bloodstream and facilitates its uptake into cells. Additionally, the 2'-MOE modifications reduce the immunogenicity of the oligonucleotide, minimizing the risk of adverse immune responses.
The chemical modifications in Eplontersen are essential for its therapeutic efficacy and safety. The phosphorothioate backbone provides stability and protection against nuclease degradation, ensuring that the drug remains active and effective in the cellular environment. The 2'-MOE modifications enhance binding affinity and reduce off-target effects, ensuring that the drug specifically targets the TTR mRNA. Together, these modifications improve the pharmacokinetic and pharmacodynamic properties of Eplontersen, making it a potent and safe therapeutic option for the treatment of hATTR with polyneuropathy.
Summary and Prospect
In summary, Eplontersen represents a significant advancement in antisense technology and the treatment of hereditary transthyretin-mediated amyloidosis (hATTR) with polyneuropathy. Its mechanism of action, involving the degradation of TTR mRNA, has shown promising results in reducing TTR levels and improving neurological function in affected patients. Despite facing competition from other RNA-targeted therapies, Eplontersen offers a unique advantage with its subcutaneous administration, which may improve patient convenience and compliance. Future research aimed at improving delivery methods and expanding the range of treatable conditions could further enhance its clinical utility. The sequence characteristics and chemical modifications of Eplontersen, including its phosphorothioate backbone and 2'-MOE modifications, contribute to its stability, specificity, and efficacy, making it a valuable tool in the management of hATTR.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
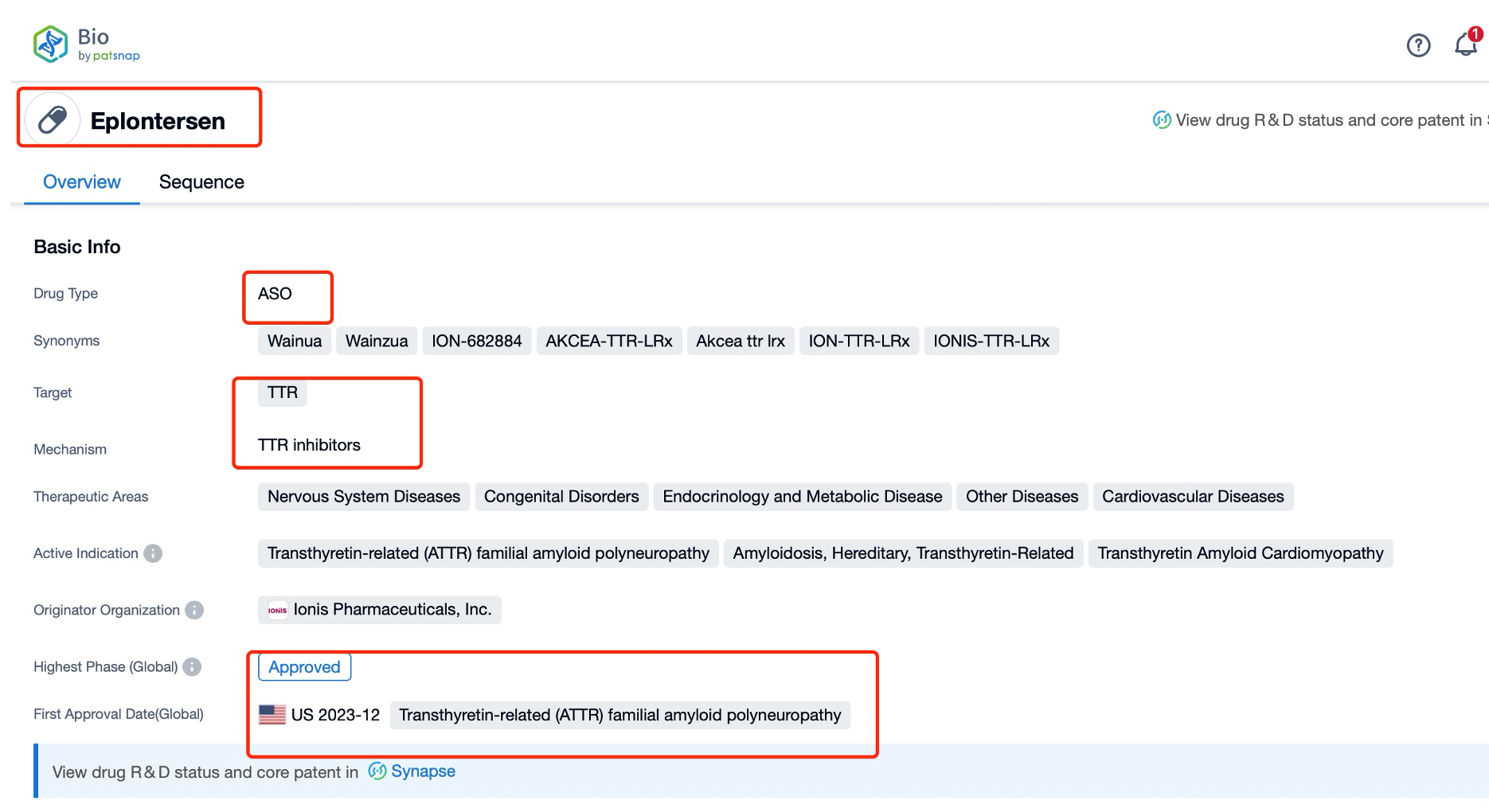
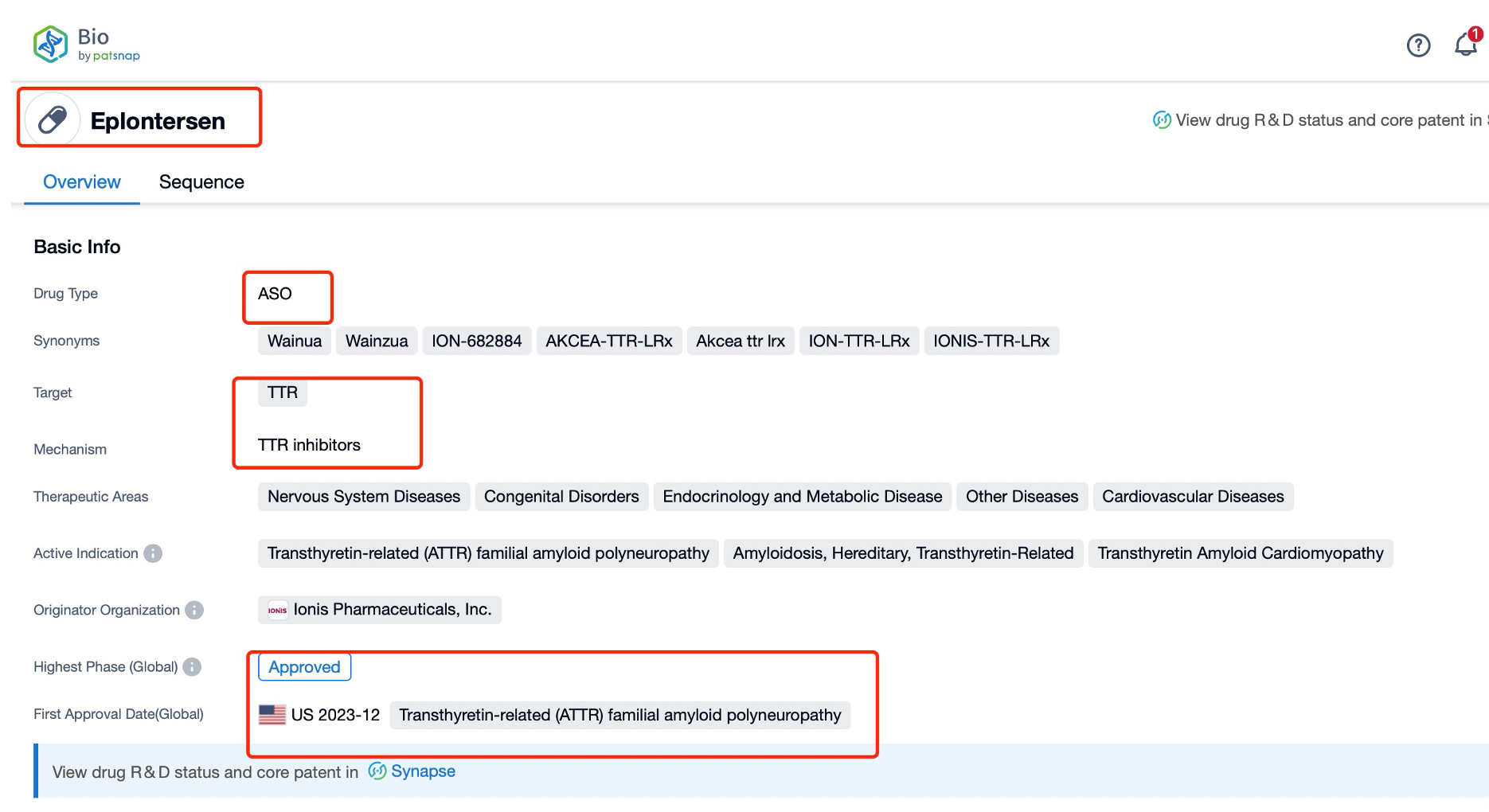
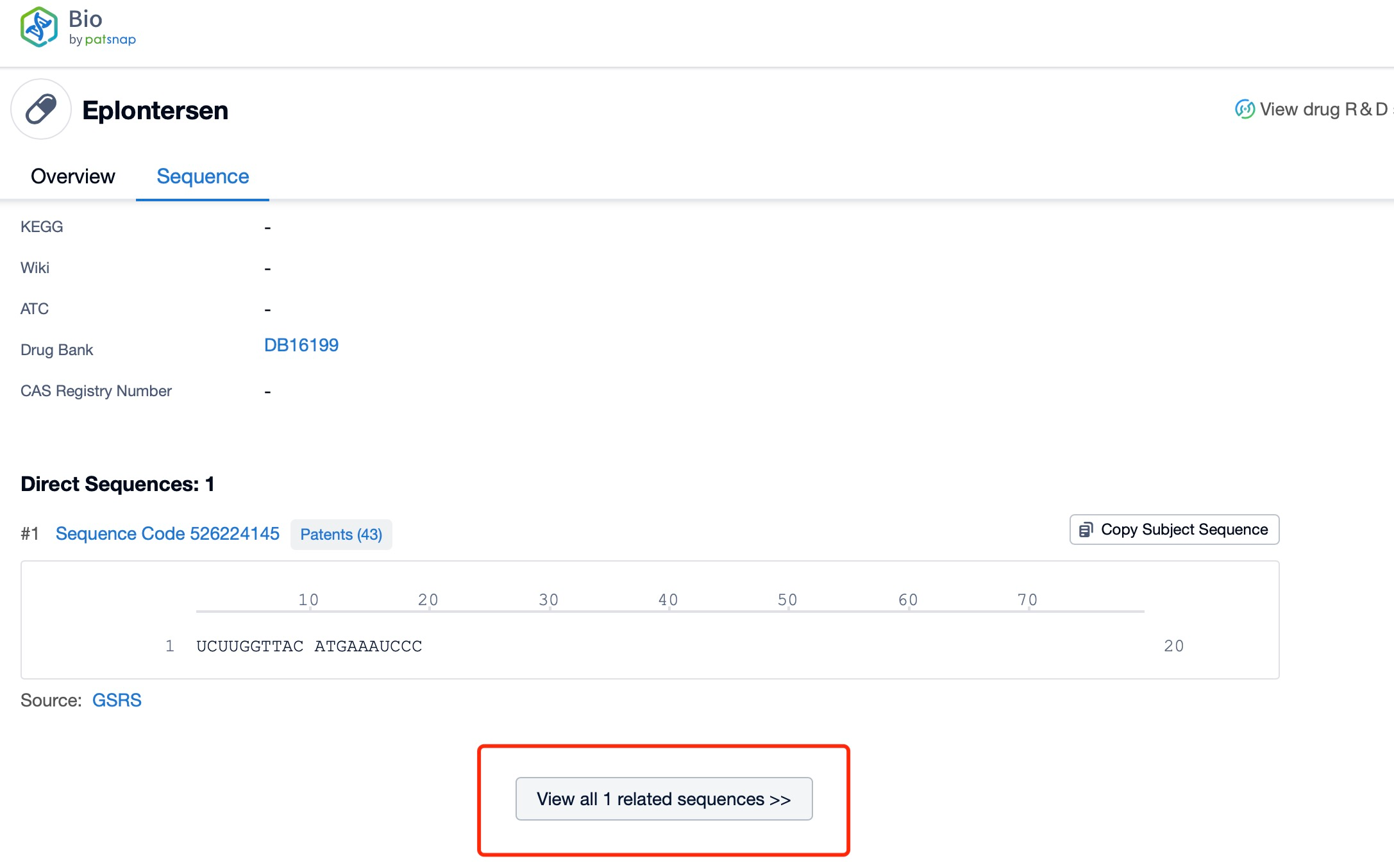
Taking Eplontersen as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Eplontersen ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Eplontersen.
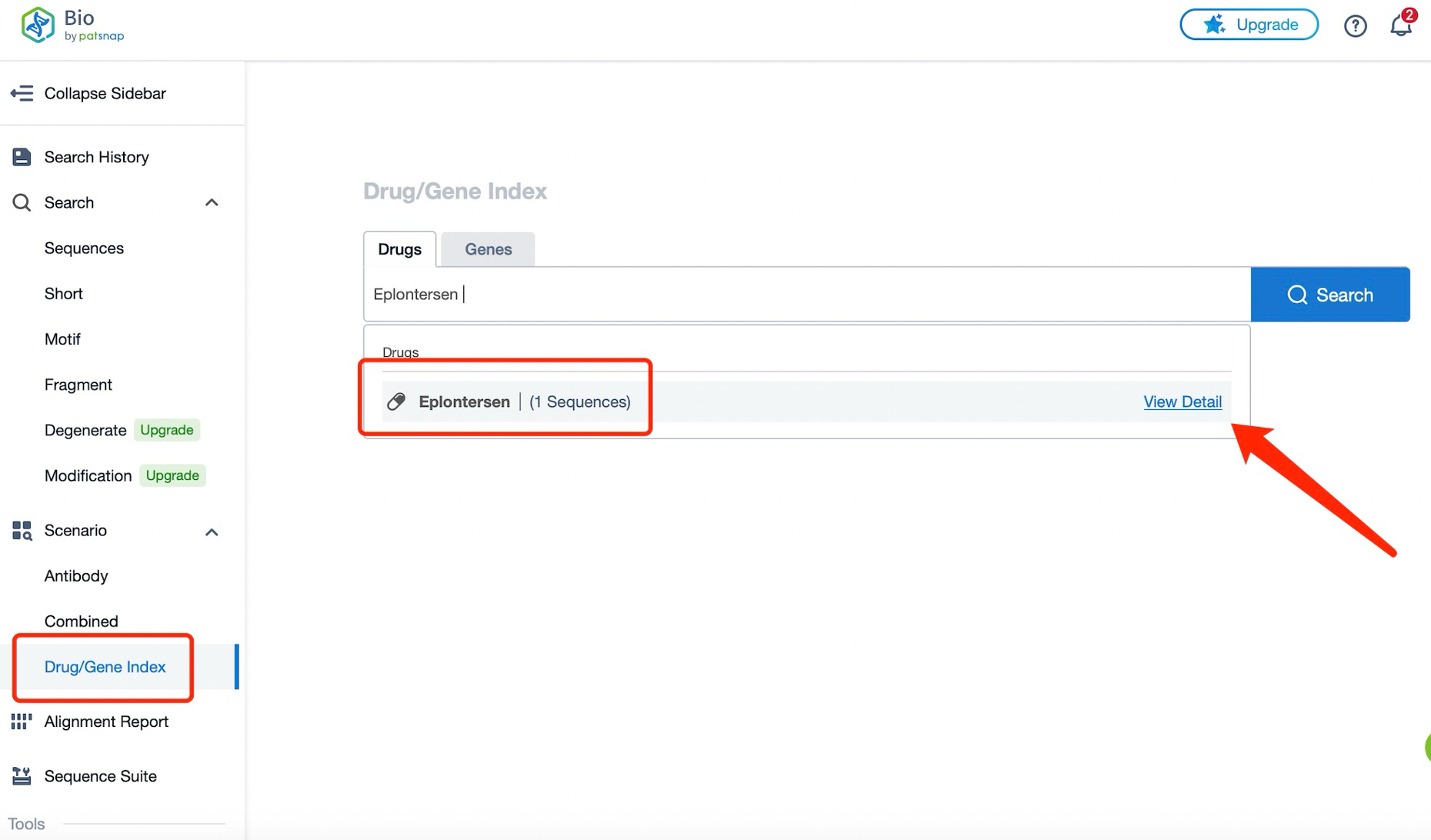
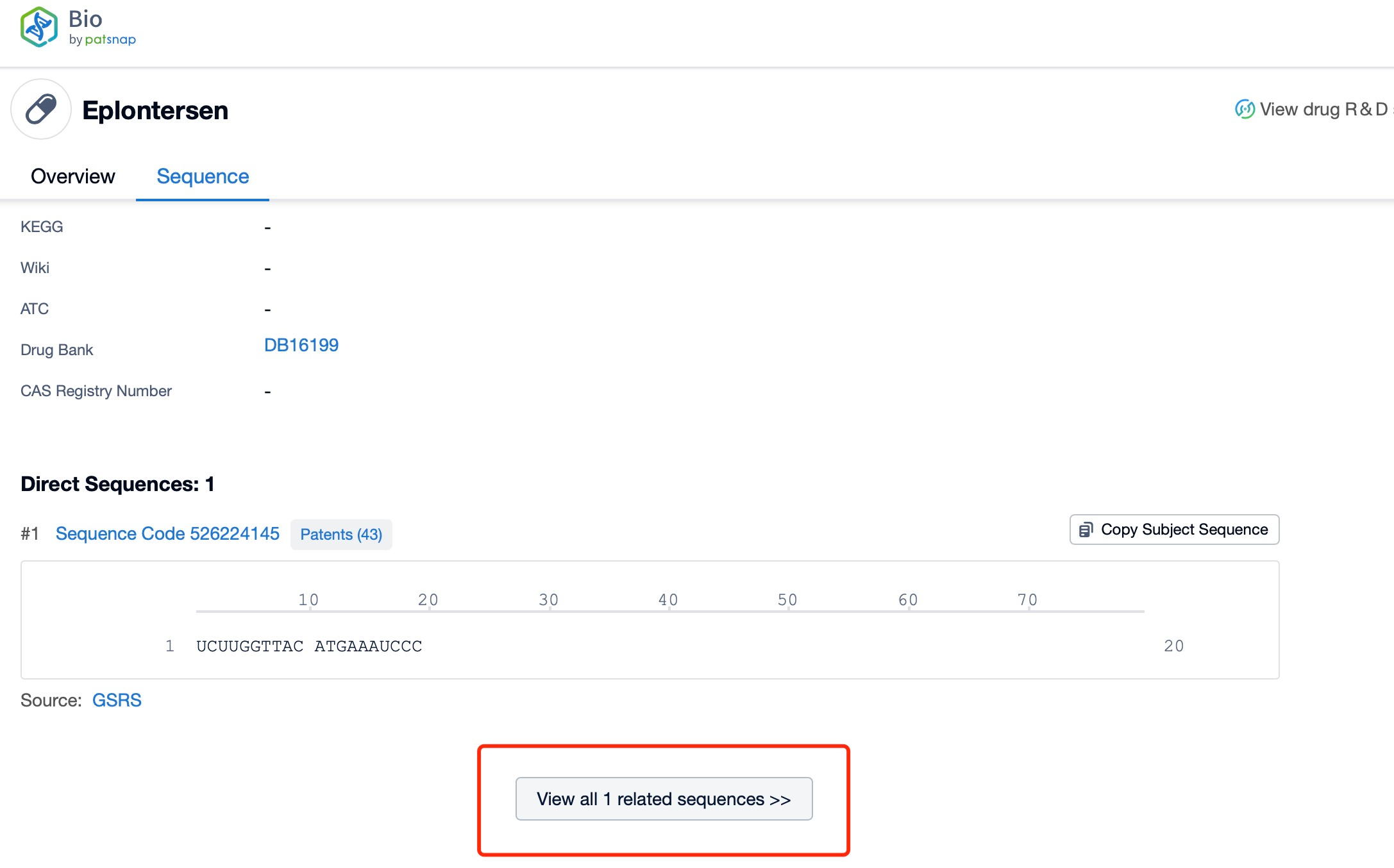
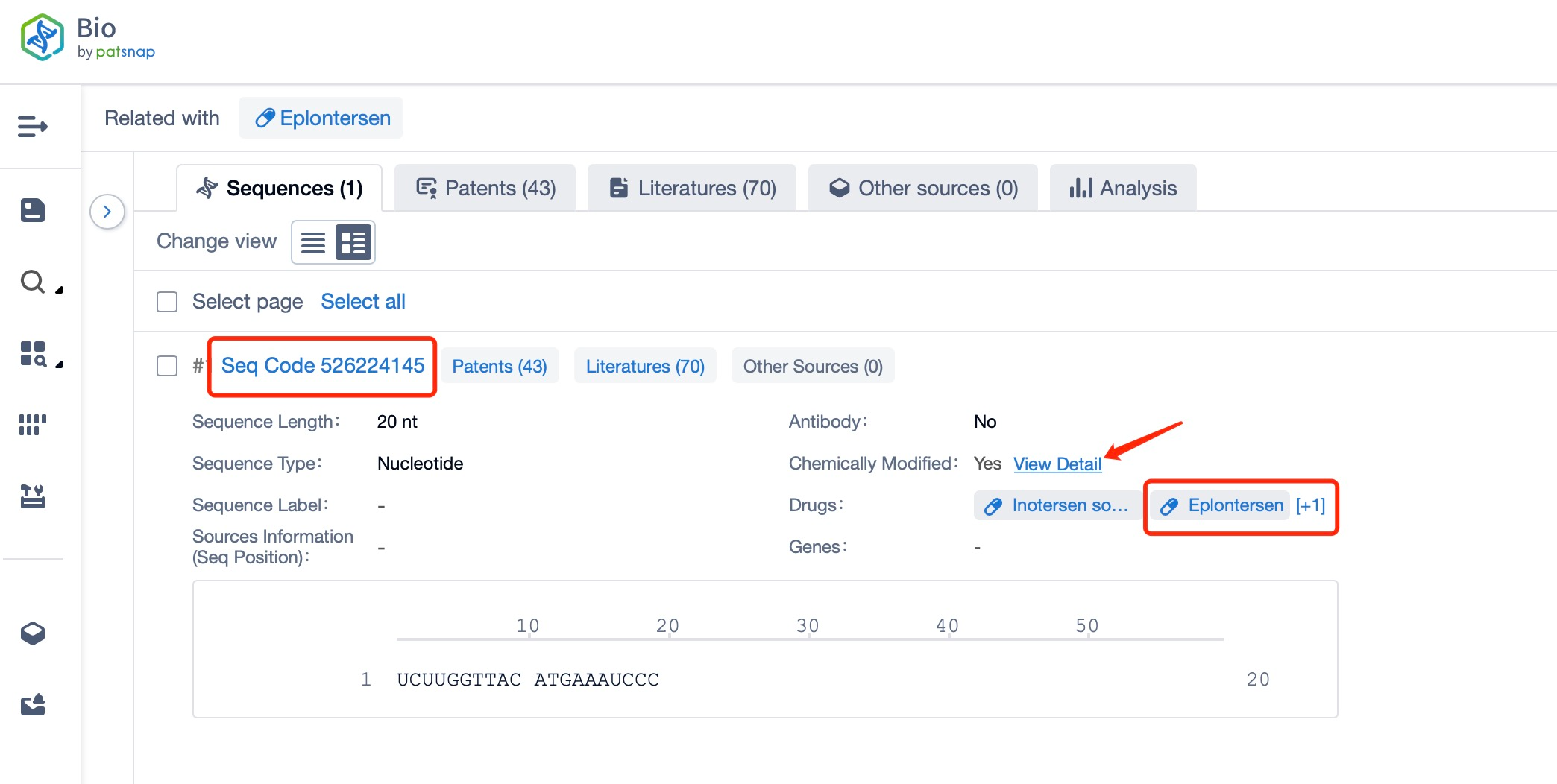
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.