Pfizer Reveals Key Findings of ABRYSVO® for RSV in Adults with Compromised Immunity
Pfizer Inc. (NYSE: PFE) has revealed encouraging top-line outcomes regarding safety and immunogenicity from substudy B of its ongoing pivotal Phase 3 clinical trial (NCT05842967) known as MONeT (RSV IMmunizatiON Study for AdulTs at Higher Risk of Severe Illness). This research is assessing the administration of two doses of the ABRYSVO vaccine in immunocompromised adults aged 18 and above who are at an increased risk of developing severe lower respiratory tract disease (LRTD) connected to respiratory syncytial virus (RSV).
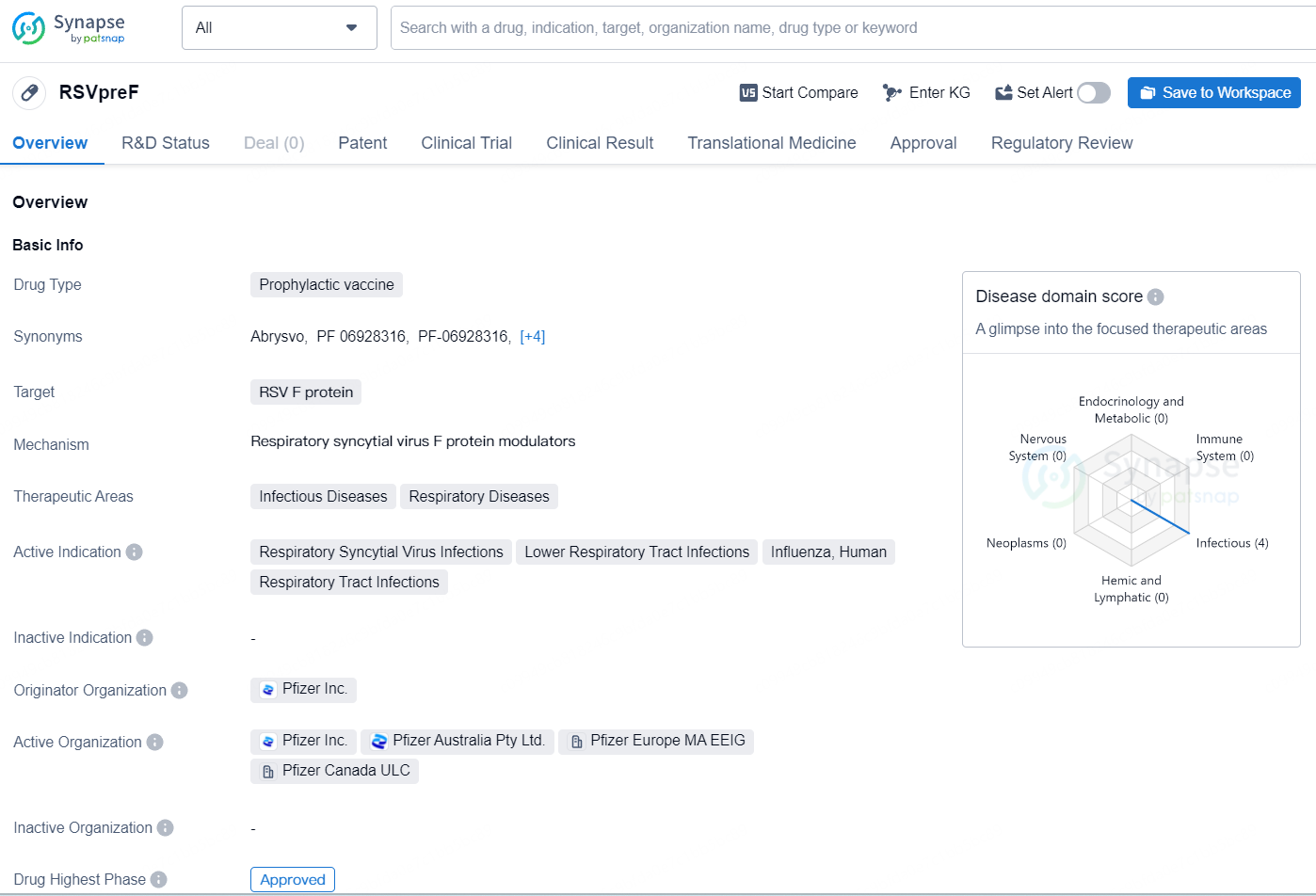
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Individuals with immunocompromising conditions are at an elevated risk of acquiring RSV-LRTD. Substudy B of the MONeT trial evaluated the safety and immunogenicity of two ABRYSVO doses, given a month apart, in four cohorts of immunocompromised adults: those with non-small cell lung cancer, those on hemodialysis due to end-stage renal disease, those with autoimmune inflammatory disorders on active immunomodulator therapy, and recipients of solid organ transplants. Among the 203 participants enrolled in the substudy, around half were aged 18 to 59, while the other half were 60 years or older.
During the trial, ABRYSVO was well-tolerated, with a safety profile consistent with previous studies. Although two doses of the vaccine were assessed, a single 120 µg dose of ABRYSVO induced a robust neutralizing response against both RSV subtypes, RSV-A and RSV-B, in all cohorts and age groups. Pfizer intends to present these findings at an upcoming scientific conference, publish them in a peer-reviewed journal, and submit the data to regulatory authorities for review.
“Immunocompromised adults, including those with cancer or autoimmune disorders, face a significantly higher risk of severe complications from RSV. Currently, no vaccines are approved for individuals aged 18 to 59 in the U.S.,” stated Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer. “We are encouraged by the positive top-line data from this study, which provide critical evidence that ABRYSVO could meet a significant unmet need in this vulnerable population.”
These latest findings in immunocompromised adults enhance the existing evidence supporting ABRYSVO’s profile in high-risk adults. In June 2024, during the Advisory Committee on Immunization Practices (ACIP) meeting, Pfizer presented results from a cohort of adults aged 18-59 with specific chronic medical conditions. In this double-blinded study, 681 adults aged 18 to 59 with chronic conditions were randomized 2:1 to receive a single dose of ABRYSVO or a placebo.
Participants exhibited RSV-A and RSV-B subgroup neutralizing responses that were non-inferior to those observed in the Phase 3 (NCT05035212) RENOIR study of ABRYSVO, which had earlier demonstrated the vaccine’s efficacy in adults aged 60 and older. These results bolster earlier data presented at the ACIP highlighting ABRYSVO’s high clinical effectiveness against lower respiratory tract disease in adults aged 60 years and older, including a substantial proportion of immunocompromised individuals.
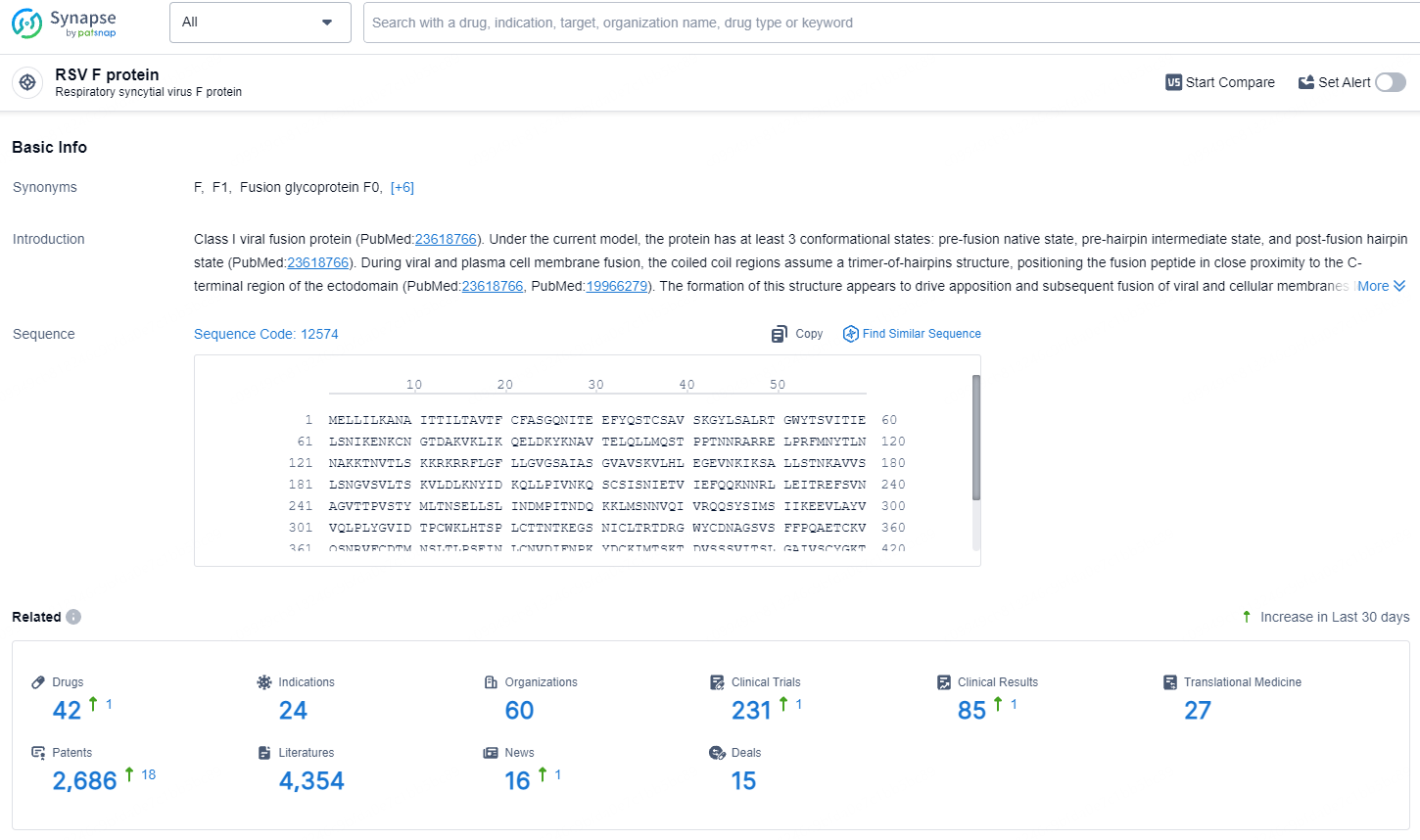
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 14, 2024, there are 767 investigational drugs for the RSV F protein targets, including 254 indications, 484 R&D institutions involved, with related clinical trials reaching 1230, and as many as 42951 patents.
RSVpreF is a prophylactic vaccine targeting the RSV F protein, designed to treat infectious and respiratory diseases such as Respiratory Syncytial Virus Infections, Lower Respiratory Tract Infections, Influenza, Human, and Respiratory Tract Infections. The originator organization of this drug is Pfizer Inc. It has reached the highest phase of approval globally and has an ongoing Investigational New Drug (IND) Application phase in China.