Halda Therapeutics Raises $126M to Progress RIPTAC™ Cancer Treatments for Key Solid Tumors into Clinical Trials
Halda Therapeutics, a biotechnology firm focusing on a groundbreaking type of cancer treatment known as RIPTAC™ (Regulated Induced Proximity Targeting Chimeras) therapeutics, has announced the securing of $126 million in their Series B extension financing. The funds will be used to push forward two RIPTAC candidates to clinical trial phases for patients suffering from prostate cancer and breast cancer, particularly in metastatic cases where resistance to conventional treatments is common. The leading RIPTAC treatment from Halda, HLD-0915, is anticipated to begin a Phase 1 clinical trial in the first half of 2025, targeting patients with metastatic, castration-resistant prostate cancer (mCRPC).
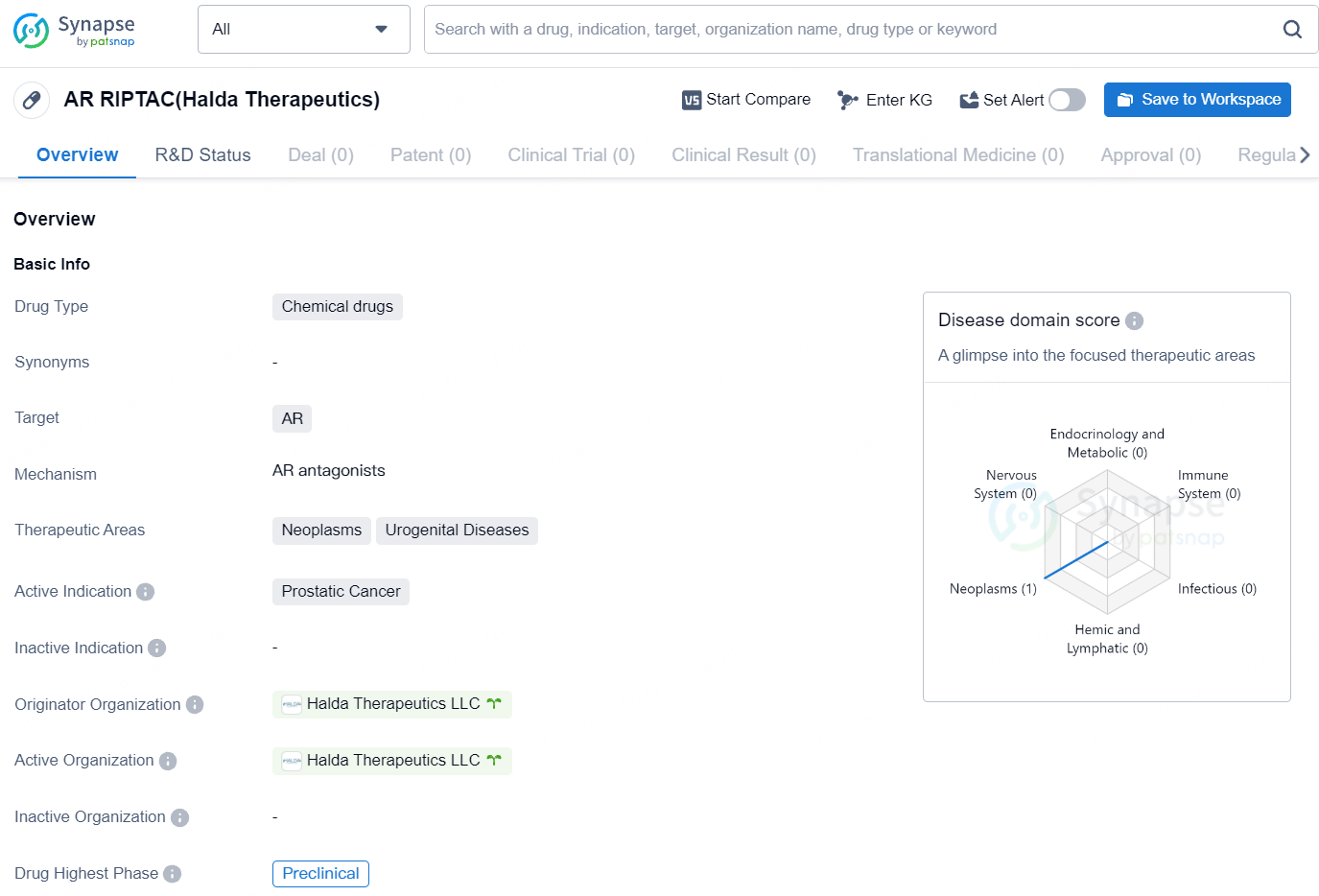
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Kat Kayser-Bricker, PhD, Chief Scientific Officer of Halda Therapeutics, expressed enthusiasm about the support from a prominent group of new healthcare investors who align with their goal to innovate in cancer drug development. This funding will facilitate their mission to deliver an oral, selective, and broadly effective cancer cell-killing mechanism designed to overcome drug resistance, a significant limitation of many current standard cancer treatments. "Our exceptional scientific team has achieved remarkable milestones, from the creation of the RIPTAC modality to the development of two promising programs targeting prostate and breast cancer. Our first drug candidate is set to enter clinical trials for mCRPC patients in early 2025," she stated.
With this financing, the RIPTAC modality is progressing into clinical trials, representing a heterobifunctional molecule developed by Halda that targets two proteins to enable a novel cancer cell-killing mechanism tailored to address specific cancer types. RIPTAC therapeutics operate through a unique “hold and kill” mechanism by bringing together a cancer-specific protein and a protein with a crucial function, resulting in the disruption of the essential cell function and the subsequent death of cancer cells, sparing non-cancerous tissues where the cancer-specific protein is either absent or minimally expressed.
Joe Cabral, Principal at Frazier Life Sciences, remarked, "Innovative mechanisms are crucial to overcoming resistance to standard of care treatments across various tumor types. RIPTAC therapies selectively eliminate cancer cells based on differential protein expression in orally available medicines. This innovation holds promise for treating advanced cancer patients with heterogeneous resistance as well as those in the earlier stages of disease. We are excited to collaborate with Halda’s scientists and clinicians to bring novel therapies to improve patient outcomes."
Arjun Goyal, MD, Co-Founder and Managing Director at Vida Ventures, praised Halda’s efforts in ushering in a new era of oncology treatment with RIPTAC therapeutics, highlighting the potential to provide solutions for patients with prevalent conditions like prostate and breast cancer. Specifically, Halda’s leading RIPTAC candidate, HLD-0915, offers a mutation-agnostic small molecule approach for mCRPC, addressing unfulfilled needs for prostate cancer patients worldwide.
As part of this financing, Rebecca Luse, Principal at Deep Track Capital, Joe Cabral, Principal at Frazier Life Sciences, Nandita Shangari, PhD, Managing Director at RA Capital Management, and Arjun Goyal, MD, Co-Founder and Managing Director at Vida Ventures, will join the Halda Board of Directors.
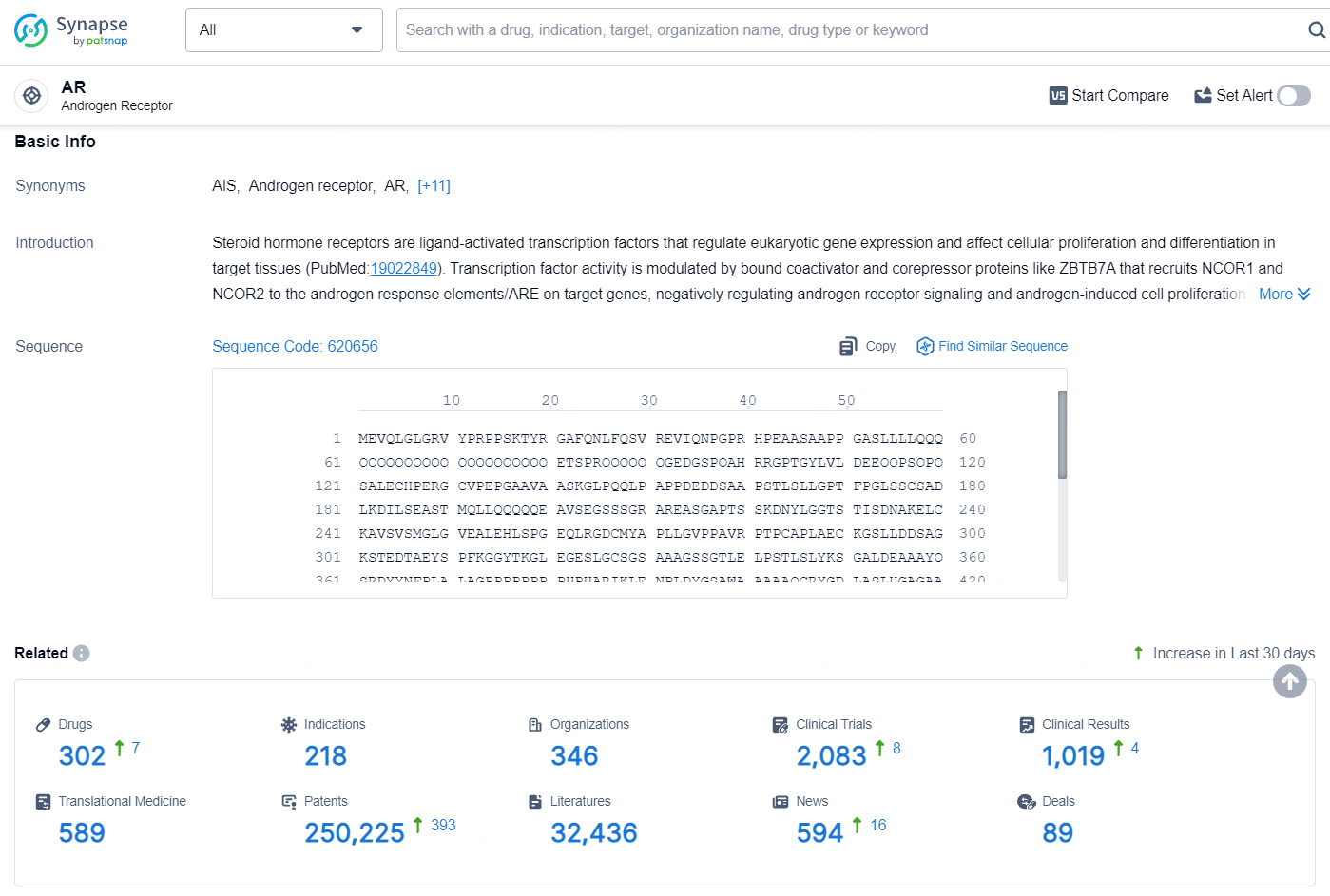
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 14, 2024, there are 302 investigational drugs for the AR target, including 218 indications, 346 R&D institutions involved, with related clinical trials reaching 2083, and as many as 250225 patents.
AR RIPTAC is a chemical drug developed by Halda Therapeutics LLC, targeting the androgen receptor (AR). It falls under the therapeutic areas of neoplasms and urogenital diseases, with a specific active indication for prostatic cancer. As of now, the drug is in the preclinical phase and is being developed by Halda Therapeutics LLC.