First U.S. Participant Joins Global Clinical Trial for Telitacicept in Myasthenia Gravis: A Milestone
RemeGen Co. Ltd., a biotech company in the commercial phase, revealed that their innovative BLyS/APRIL dual targeting fusion protein medication, telitacicept, has enrolled its first patient in the United States as part of a global multi-center phase III clinical trial aimed at treating generalized myasthenia gravis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
This landmark achievement represents a crucial phase in the global clinical advancement of telitacicept, offering renewed hope to myasthenia gravis sufferers worldwide. Originally, telitacicept received the orphan drug and fast track designations for myasthenia gravis from the US Food and Drug Administration, alongside the breakthrough therapy designation by China’s National Medical Products Administration.
This international, multicenter, randomized, double-blind, placebo-controlled, phase III trial seeks to assess the efficacy and safety of telitacicept for treating gMG. The study aims to enroll 180 patients from various countries and regions across the globe.
Myasthenia gravis is a rare, chronic autoimmune disorder that hampers neuromuscular junction transmission, potentially affecting eye movement, swallowing, speech, overall mobility, and respiratory function to varying extents. As highlighted in the Frost & Sullivan report, the worldwide myasthenia gravis patient population is projected to reach 1.15 million by 2025, with approximately 70,000 cases in the United States and 22,000 in China.
Currently, primary treatment options for myasthenia gravis include cholinesterase inhibitors, glucocorticoids, and immunosuppressants. Yet, a notable unmet medical need exists as many patients do not respond effectively due to the lack of efficacy, tolerability issues, or contraindications associated with existing treatments.
Telitacicept is a dual-targeting antibody fusion protein that inhibits BLyS and APRIL, directly targeting the source of pathogenic antibodies — B cells and plasma cells — thereby reducing the production of these antibodies and achieving its therapeutic goals. Initial clinical trials have demonstrated that telitacicept can consistently and effectively enhance the clinical condition of patients with generalized myasthenia gravis.
Since its approval in China in March 2021, telitacicept has benefited over 40,000 patients, exhibiting excellent efficacy and safety. In addition to its approved indications for systemic lupus erythematosus and rheumatoid arthritis in China, telitacicept may receive approval for myasthenia gravis, Sjogren’s syndrome, IgA nephritis, and neuromyelitis optica in the forthcoming years.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
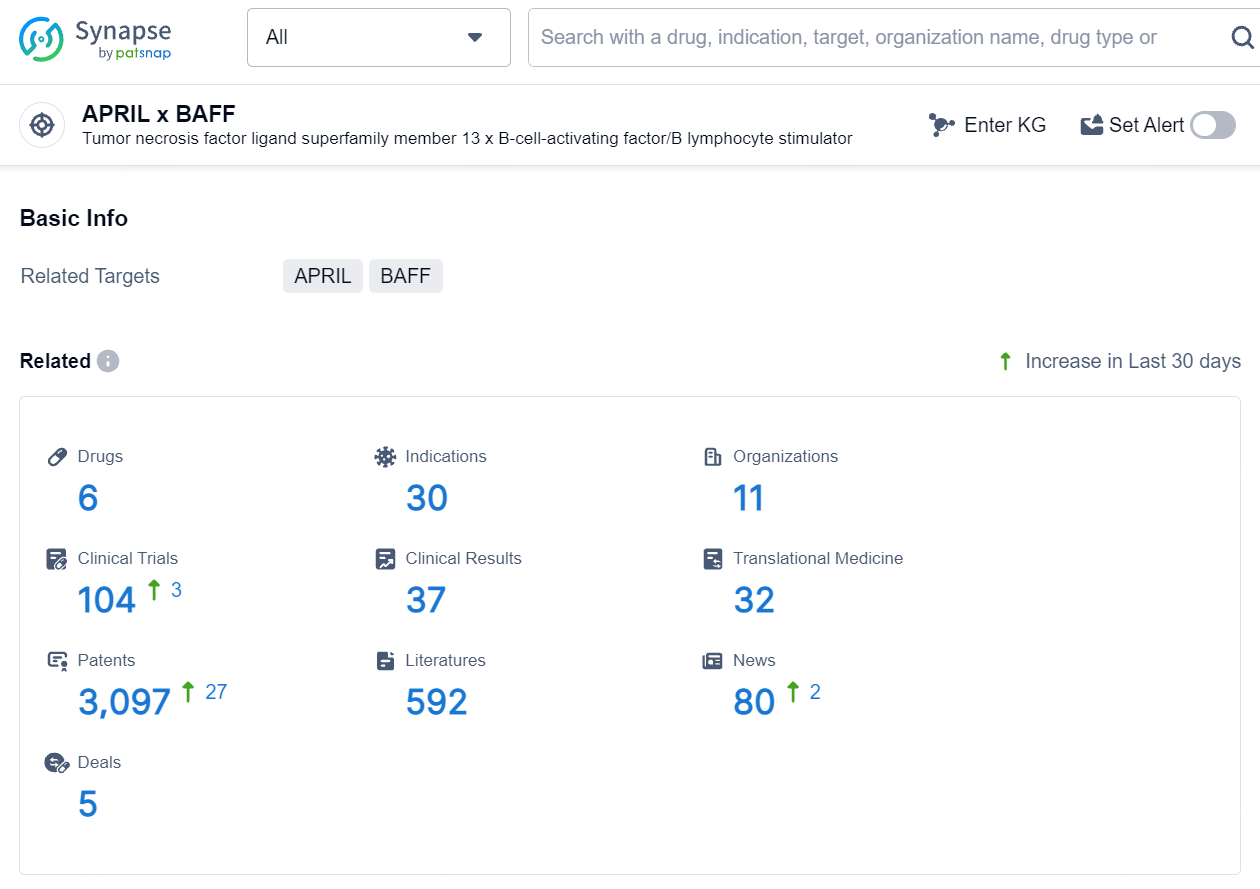
According to the data provided by the Synapse Database, As of August 9, 2024, there are 6 investigational drugs for the APRIL and BAFF target, including 30 indications, 11 R&D institutions involved, with related clinical trials reaching 104, and as many as 3097 patents.
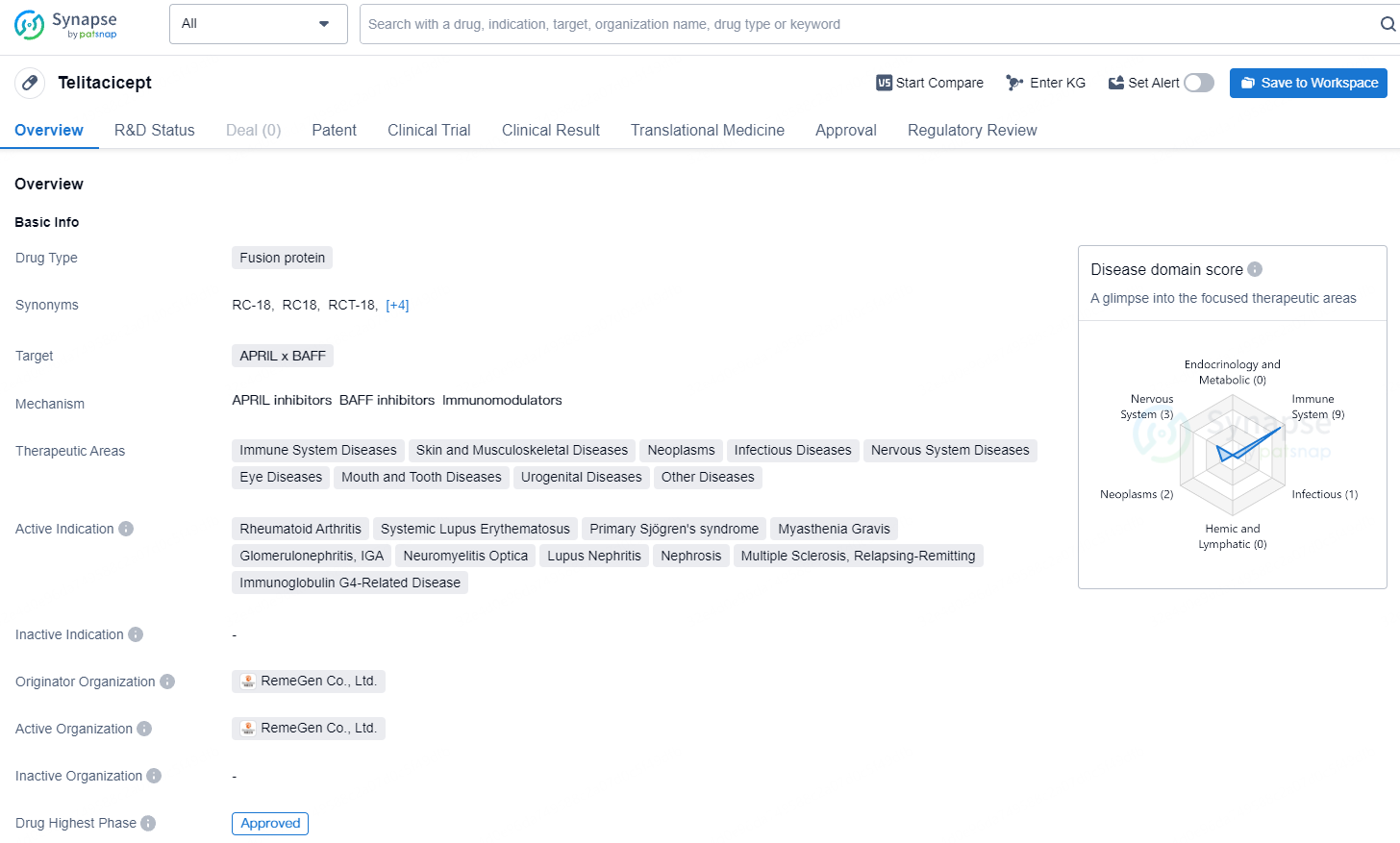
Telitacicept is a fusion protein drug targeting APRIL x BAFF and has been approved for various therapeutic areas and active indications, with its highest phase of approval achieved globally and in China. The drug, developed by RemeGen Co., Ltd., received its first approval in China in March 2021 and has been granted several regulatory designations.