GC Biopharma and Novel Pharma Initiate Global Phase I Trial for MPS IIIA Treatment
GC Biopharma and Novel Pharma declared that the initial patient in the United States has received treatment in a multinational clinical study involving GC1130A, a novel therapeutic agent aimed at addressing Sanfilippo syndrome type A (MPS IIIA).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
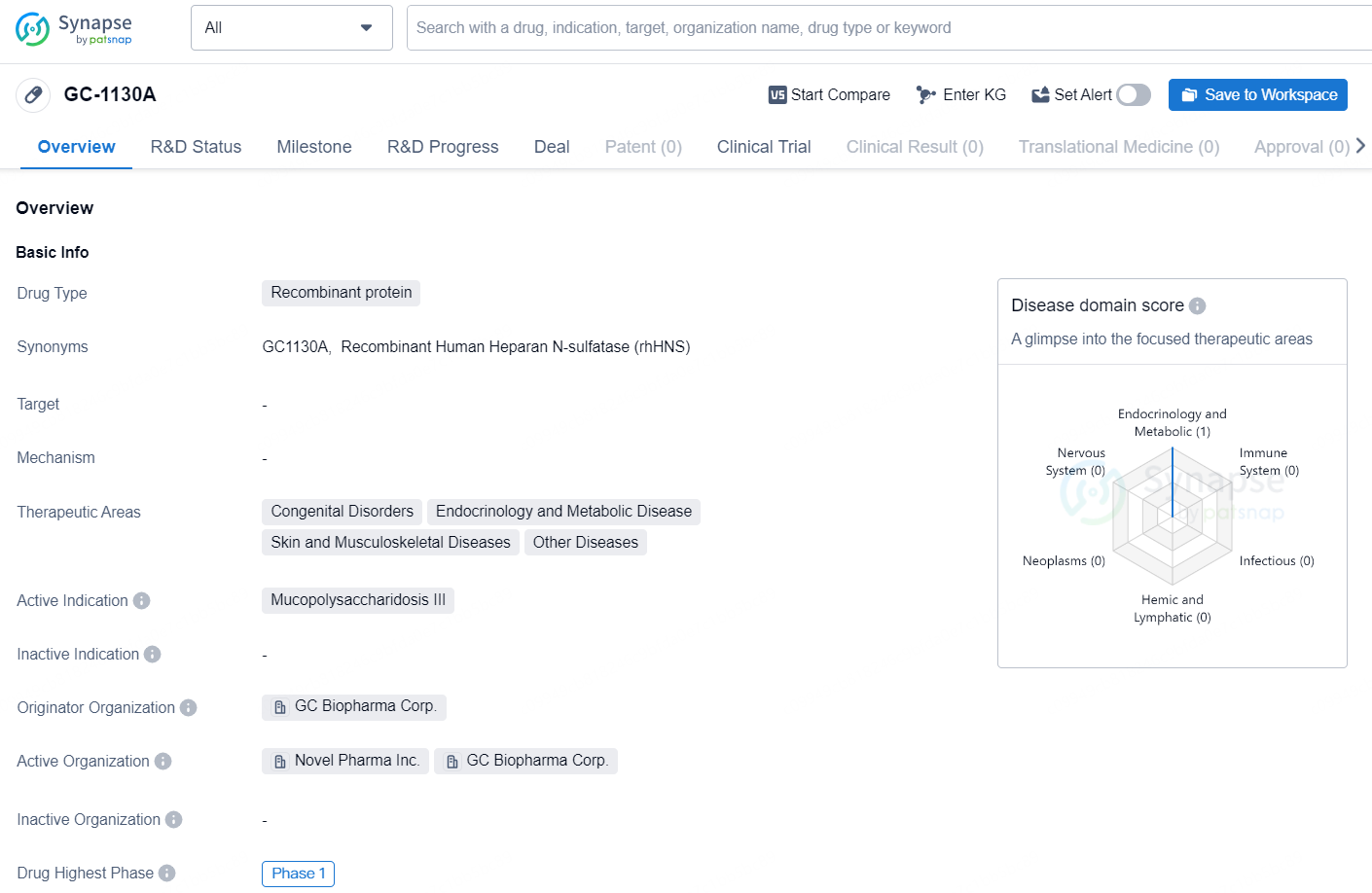
GC Biopharma and Novel Pharma have obtained IND approval for a Phase I clinical trial of GC1130A in the United States, South Korea, and Japan, and are now executing a multinational trial. This Phase I study aims to assess the safety, tolerability, and efficacy of GC1130A over a two-year timeline for pediatric patients aged two to six years who are diagnosed with Sanfilippo syndrome type A (MPS IIIA).
The trial will take place at two to three sites in the U.S., including the University of California San Francisco (UCSF) Benioff Children’s Hospitals, as well as at Samsung Medical Center and Ajou University Hospital in South Korea, along with one site in Japan. Eligible patients identified during the screening process will undergo surgery to have an intracerebroventricular (ICV) access device implanted, followed by biweekly administration of GC1130A.
Sanfilippo syndrome type A (MPS IIIA) is an autosomal recessive disorder characterized by central nervous system damage caused by heparan sulfate buildup. The primary clinical manifestation is cognitive deficits, and this condition is fatal, typically leading to death by approximately age 15. Given the absence of effective treatments, there is a significant unmet medical need.
GC1130A is a pioneering treatment that employs GC Biopharma's unique platform to create high-quality, concentrated recombinant proteins, which are delivered through ICV injection to circumvent the blood-brain barrier. GC Biopharma previously developed the first enzyme replacement therapy (ERT) administered ICV for Hunter syndrome, marketed in Japan as Hunterase ICV.
In preclinical research, GC Biopharma demonstrated that ICV delivery is up to 47 times more effective than intrathecal (IT) administration.
SooKyung Shin, Head of the Medical Division at GC Biopharma, stated, “The start of patient dosing in the United States marks a significant achievement for the global clinical trials of ‘GC1130A,’ and we are committed to accelerating the clinical development process.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 4, 2024, there are 38 investigational drugs for the Mucopolysaccharidosis II, including 6 targets, 33 R&D institutions involved, with related clinical trials reaching 79, and as many as 2227 patents.
GC-1130A is a recombinant protein drug developed by GC Biopharma Corp. The drug falls under the therapeutic areas of Congenital Disorders, Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, and Other Diseases. The active indication for GC-1130A is Mucopolysaccharidosis III, also known as Sanfilippo Syndrome, which is a rare genetic disorder that affects the metabolism of complex sugar molecules.