Genentech's Vabysmo gets FDA authorization for use in treating Retinal Vein Occlusion (RVO)
Genentech, part of the Roche Group, has communicated that the FDA has given approval for Vabysmo®(faricimab-svoa) to be used in treating macular edema that occurs after retinal vein occlusion. RVO is the third approved indication for Vabysmo, which is also utilized for wet, alternatively known as neovascular, age-related macular degeneration and diabetic macular edema. Combined, more or less three million people in the U.S. are affected by these three retinal conditions, which are prominent factors in causing vision loss.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Levi Garraway, M.D., Ph.D., Chief Medical Officer of Genentech and Head of Global Product Development, highlighted that Vabysmo is an innovative solution for RVO patients, with potential to not only help preserve vision but also to improve it, whilst providing the added perk of retinal drying. He asserted that the effectiveness and safe usage of Vabysmo is well-documented in international clinical tests, which is further supported by a wealth of practical evidence, with this treatment being used for hundreds of thousands of individuals.
Vabysmo stands alone as the first approved bispecific antibody specifically for the eye. The sanctioning for RVO use is underpinned by promising results from the international phase III BALATON and COMINO studies. These demonstrated that monthly administration of Vabysmo delivered prompt and prolonged enhancement of vision for patients with both branch and central RVO. This conclusively met the primary endpoint of equaling the visual acuity gains at 24 weeks as compared to aflibercept.
The data was further corroborated by results showing that Vabysmo was successful in quickly and effectively drying retinal fluid. The BALATON and COMINO studies found that Vabysmo was generally well-received by patients, with its safety parameters aligning with previous tests. Conjunctival hemorrhage was a common side effect. The safety outcomes remained consistent across all study arms.
Alarm bells were added to the Warnings & Precautions section of the American label, given infrequent cases of retinal vasculitis and/or retinal vascular occlusion identified after its market release usually when there was intraocular inflammation. The incidence rate for retinal vasculitis with vascular occlusion is 0.06 per 10,000 injections, aligning with the real-world incident rates of other widely used intravitreal treatments.
Till now, Vabysmo has obtained approval in over 80 countries globally, supporting individuals diagnosed with wet AMD and DME. Vabysmo has been distributed approximately 2 million times worldwide.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
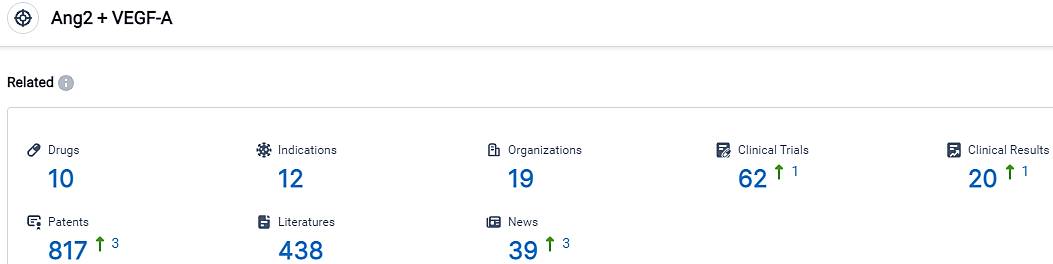
According to the data provided by the Synapse Database, As of October 30, 2023, there are 10 investigational drugs for the Ang2 and VEGF-A target, including 12 indications, 19 R&D institutions involved, with related clinical trials reaching 62,and as many as 817 patents.
Vabysmo is the initial dual-specific antibody granted approval for ocular use. It neutralizes angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A), inhibiting two signal pathways associated with several retinal conditions that can endanger vision. Investigations are ongoing to comprehend the role of Ang-2 pathway in retinal diseases comprehensively. However, it's hypothesized that Ang-2 and VEGF-A contribute to vision impairment by causing instability in the blood vessels, which can potentially lead to the formation of new permeable vessels and heightened inflammation.