Tenaya Therapeutics receives FDA approval to initiate the clinical trial of TN-401 gene therapy for treating PKP2-related Arrhythmogenic Right Ventricular Cardiomyopathy
Tenaya Therapeutics, Inc., a biotechnology organization in the clinical phase that aims to identify, advance, and potentially provide curative treatments that target the fundamental triggers of heart disease, declared today that they have received approval from the FDA for their IND application to start clinical trials for TN-401.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TN-401 is Tenaya’s adeno-associated virus serotype 9 -based investigational gene therapy product TN-401 is an adeno-associated virus serotype 9 based gene therapy product under investigation by Tenaya, formulated for the treatment of arrhythmogenic right ventricular cardiomyopathy induced by abnormalities in the plakophilin-2 gene.
On receiving clearance for the IND, Tenaya plans to commence the RIDGE-1™ Phase 1b clinical trial for TN-401, consisting of a multi-center, open-label study for evaluating the safety, acceptability, and clinical effectiveness of a single intravenous injection of TN-401. The RIDGE™ international observational natural history and serotype study of PKP2-linked ARVC is currently being executed by Tenaya.
ARVC stands for arrhythmogenic cardiomyopathy, a chronic, evolutionary, inherited disorder typically showing up before 40 years of age. Those affected with ARVC exhibit symptoms of ventricular arrhythmias, like palpitations, faintness, and light-headedness, and the risk of sudden cardiac death is higher. The genetic mutations of PKP2 are the predominant cause of ARVC and result in the loss of vital proteins required to sustain the structural stability and signaling between heart muscle cells.
TN-401 is devised to address the fundamental cause of the disease by delivering a completely functional PKP2 gene in order to restore the usual PKP2 protein levels and hence slowing down the disease progression and reversing the course of disease after a single dosage.
“Our patients with arrhythmogenic cardiomyopathy experienced massive levels of anxiety and stress and they need to endure the aggravating physical and lifestyle constraints to manage the frequently abnormal heart rhythms and perpetual risk of sudden cardiac arrest associated with this disease.” commented Whit Tingley, Tenaya’s Chief Medical Officer.
“TN-401 aims to tackle the genetic anomaly that is most often the cause of ARVC. We found that the initial dose of TN-401 in our RIDGE-1 study demonstrated near-maximal efficacy. As the engagement activities with clinical site and patient community are in full swing, we expect to rapidly move into the clinical trial phase." Whit Tingley concluded.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
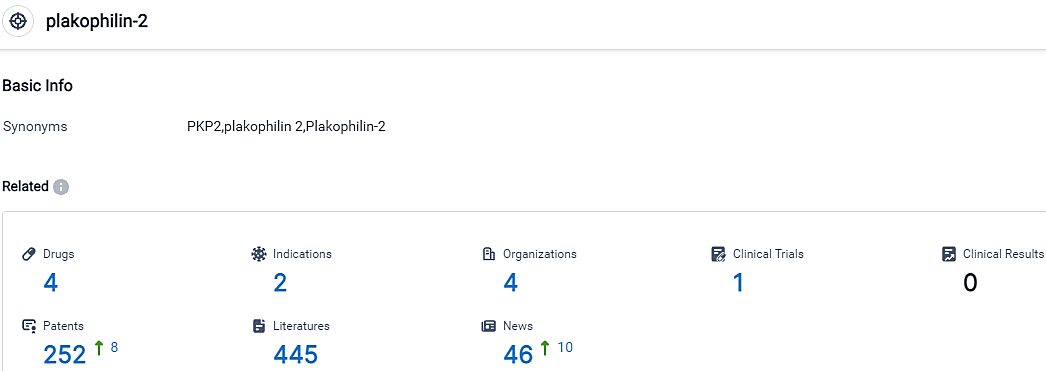
According to the data provided by the Synapse Database, As of October 30, 2023, there are 4 investigational drugs for the plakophilin-2 target, including 2 indications, 4 R&D institutions involved, with related clinical trials reaching 1,and as many as 252 patents.
Tenaya has accomplished cGMP pharmaceutical production for TN-401 at the 1000-liter scale at the firm’s Genetic Medicines Manufacturing Center, with ample medication manufactured to sustain the entire Phase 1b research. The firm is now inviting participation for the international non-interventional RIDGETM study to gather natural history and AAV9 antibody details from ARVC patients who carry PKP2gene mutations. TN-401 has obtained Orphan Drug Designation from the FDA.