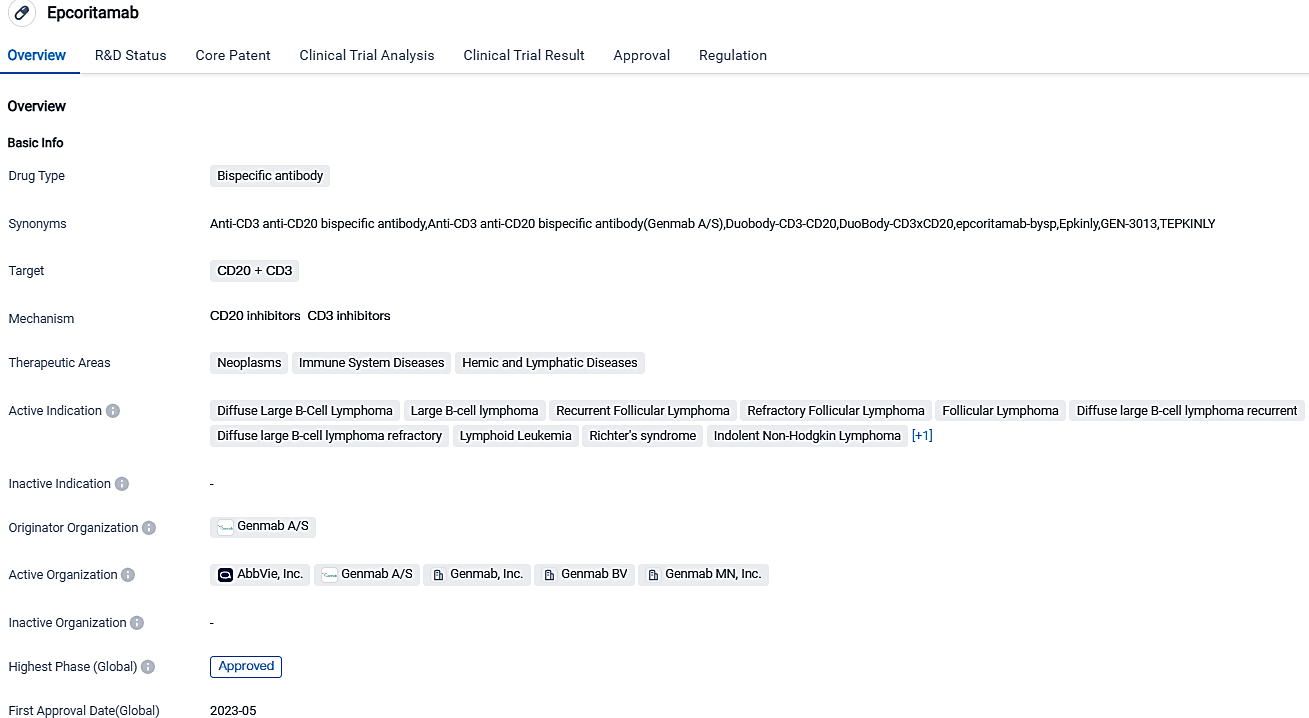
Genmab announces EU approval for TEPKINLY® (epcoritamab) to treat adult R/R DLBCL
Genmab A/S disclosed that TEPKINLY(epcoritamab) received conditional marketing approval from the EC. This authorization purposes the drug's use as a standalone treatment for adult patients who have relapsed or refractory DLBCL, post the completion of two or more cycles of systemic therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TEPKINLY, the unique T-cell engaging bispecific antibody that's administered subcutaneously, is the pioneer treatment authorized for this group of patients within Europe, as well as in Iceland, Liechtenstein and Norway. Having the highest incidence globally, Diffuse Large B-Cell Lymphoma (DLBCL) is a B-cell variety of non-Hodgkin’s lymphoma. While patients could leverage chemoimmunotherapy protocols for their ailment, they encounter restricted options for treatment, where a paucity of ready-to-use medicines is particularly evident for those cases where the disease is unresponsive or returns after initial therapy.
The introduction of TEPKINLY provides a new and innovative treatment option for people across Europe grappling with DLBCL that has returned or has demonstrated resistance to treatment, says Genmab's Chief Executive Officer, Jan van de Winkel, Ph.D. Anna Sureda, M.D., Ph.D., leader of the clinical hematology department, noted that relapsed or refractory DLBCL is an aggressive form of cancer contributing to a challenging healing process filled with emotional stress. At this juncture, patients may have undergone various lines of therapy and are likely to have already experienced remission. Hence, the authorization from the European Commission marks a significant milestone for the DLBCL patient community, potentially offering a more efficient way of managing a condition with limited treatment options currently available.
Epcoritamab is jointly developed by AbbVie and Genmab under their oncology partnership. In terms of commercial responsibilities, they would be shared between the two companies in the U.S. and Japan, with AbbVie spearheading additional international commercial operations. The pursuit of approval for epcoritamab across international markets will persist throughout the year on part of AbbVie.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
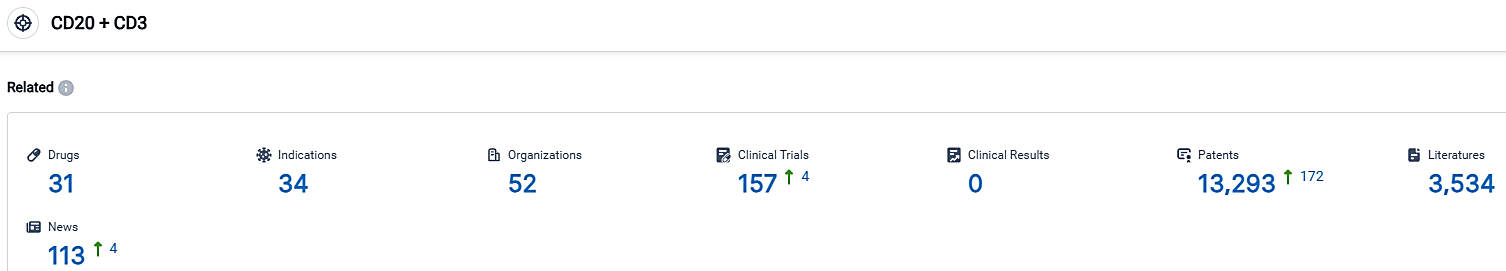
According to the data provided by the Synapse Database, As of September 29, 2023, there are 31 investigational drugs for the CD20 and CD3 target, including 34 indications,52 R&D institutions involved, with related clinical trials reaching 157,and as many as 13293 patents.
Epcoritamab is engineered to concurrently latch onto CD3 present on T-cells and CD20 on B-cells, leading to T-cell induced destruction of CD20+ cells.®v CD20, a protein found on B-cells, is a therapy target that has been clinically verified for several B-cell cancers, such as DLBCL, follicular lymphoma, mantle cell lymphoma, and chronic lymphocytic leukemia. This medication obtained expedited approval in the United States by May 2023. The consistency of this approval relies upon substantiation and delineation of its medical advantage in a subsequent confirmatory trial.