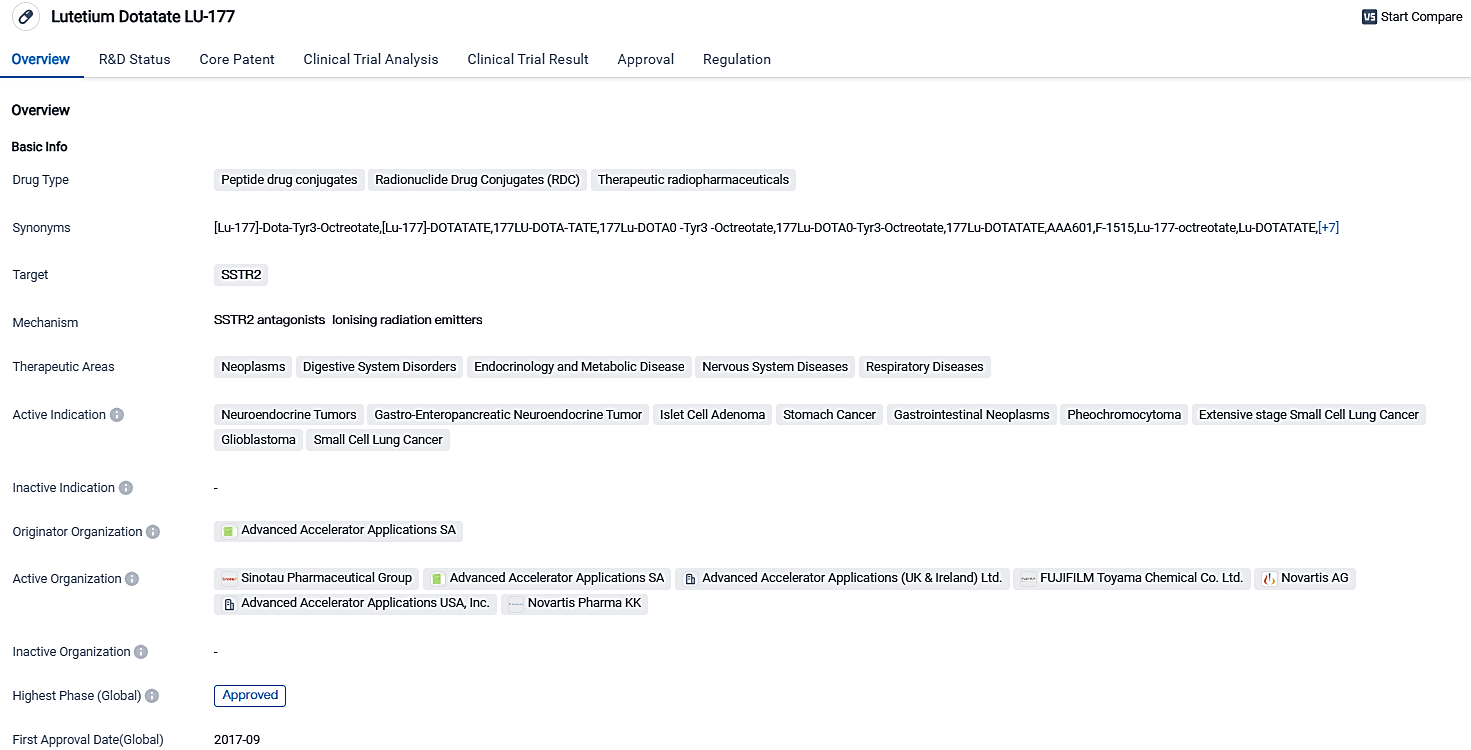
Lutathera®, a Novartis radioligand therapy, significantly improved progression-free survival rates in early advanced GEP-NETs
Novartis disclosed that the third Phase of the NETTER-2 clinical trial involving Lutathera (INN: lutetium (®177Lu) oxodotreotide / USAN: lutetium Lu 177 dotatate) has reached its fundamental goal. When used as the primary treatment in conjunction with long-acting octreotide, Lutathera showed substantial progress in PFS for patients newly diagnosed with somatostatin receptor-positive, advanced neuroendocrine tumors of Grade 2 and 3 in the gastroenteropancreatic area, in comparison to the use of high-dose long-acting octreotide by itself.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study did not reveal any new or unexpected safety concerns and the data aligns with the long-established safety record of Lutathera. Neuroendocrine tumors (NETs) are a form of cancer that develops in neuroendocrine cells spread across the body. They are typically deemed slow-growing cancers. However, some of these tumors escalate quickly and foretell a poor prognosis, with diagnosis often delayed until the patient's condition has reached an advanced stage. Despite being a rare disease, the incidence of NETs has increased by over 500% in the last 30 years, making additional treatment options for patients newly diagnosed with non-operable or advanced disease urgently needed.
The results from NETTER-2 make it the second Phase III trial in which Lutathera has presented results that are clinically significant for patients. The initial approval of Lutathera was grounded in the pivotal NETTER-1 trial. This trial revealed significant and meaningful prolongation of progression-free survival (PFS) in patients treated with Lutathera in conjunction with a long-acting dose of octreotide compared to a high-dose of long-acting octreotide alone, for patients with SSTR-positive, non-operable midgut neuroendocrine tumors who were progressing despite traditional treatment.
Jeff Legos, Executive Vice President and Global Head of Oncology Development at Novartis, expressed that these positive outcomes for Lutathera are extraordinary and showcase the potential of radioligand therapy to make a significant difference for newly diagnosed patients dealing with advanced GEP-NETs. Jeff Legos further commented that their larger, collective mission includes exploring the application of radioligand therapies as earlier treatment options for cancer patients. This is expected to help deliver ground-breaking treatment methods directly to cancer cells, potentially improving patient outcomes.
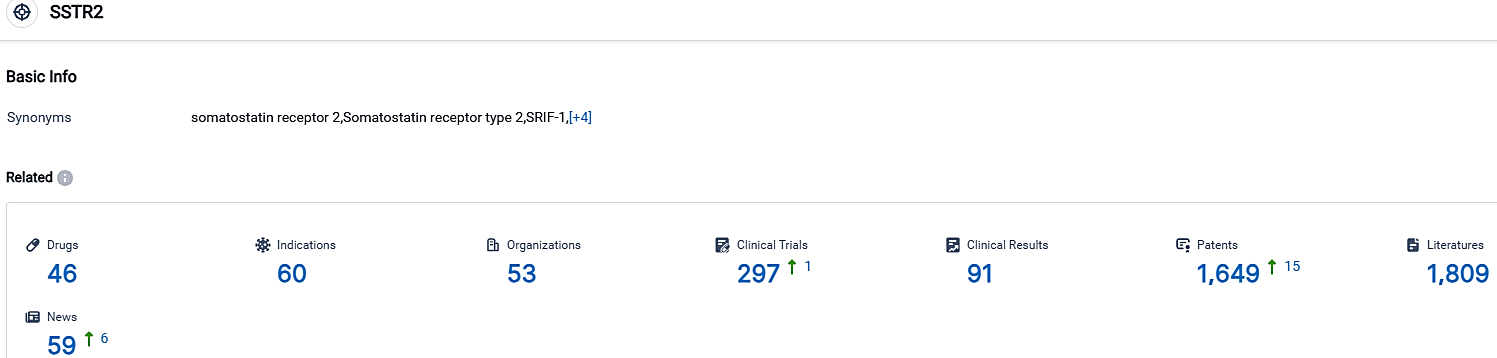
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 29, 2023, there are 46 investigational drugs for the SSTR2 target, including 60 indications,53 R&D institutions involved, with related clinical trials reaching 297,and as many as 1649 patents.
Lutathera, a Radioligand Therapy (RLT) product from Advanced Accelerator Applications, is sanctioned in the US for managing SSTR-positive Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs), encompassing those affecting the foregut, midgut, and hindgut in adult patients. In Europe, it's approved to treat adults with unresectable or metastatic, progressive, well-differentiated SSTR-positive GEP-NETs. The medicine's scope and authorization emphasizes its potential in bridging the gap in the medical necessities within the biomedical sector.