Genmab Assumes Complete Responsibility for Acasunlimab Development Initiative
Genmab A/S revealed that it will take over full responsibilities for both the ongoing development and possible commercialization of acasunlimab. BioNTech SE has decided against taking part in the further progress of the acasunlimab initiative as per the current collaboration agreement between the companies. Any progress in the program will involve paying specific milestones as well as a tiered single-digit royalty on net sales by Genmab to BioNTech.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Genmab is preparing to commence a Phase 3 trial in the latter half of this year. Despite the promising clinical outlook for acasunlimab, BioNTech has notified Genmab that this decision aligns with its portfolio strategy. The robust collaboration in antibody research between the two companies continues, with ongoing development programs proceeding under their existing agreements, which were broadened in 2022.
"Genmab’s collaboration with BioNTech has been extremely fruitful. Together, we have highlighted acasunlimab’s potential impact on patients with metastatic non-small cell lung cancer, as shown by the favorable early outcomes shared at the 2024 American Society of Clinical Oncology Meeting," stated Jan van de Winkel, Ph.D., CEO of Genmab.
"Genmab is in an excellent position to leverage the potential of acasunlimab, and we are optimistic about advancing acasunlimab into late-stage development as our second entirely owned Genmab product alongside Rina-S. We anticipate a continued productive partnership with BioNTech on other ongoing projects."
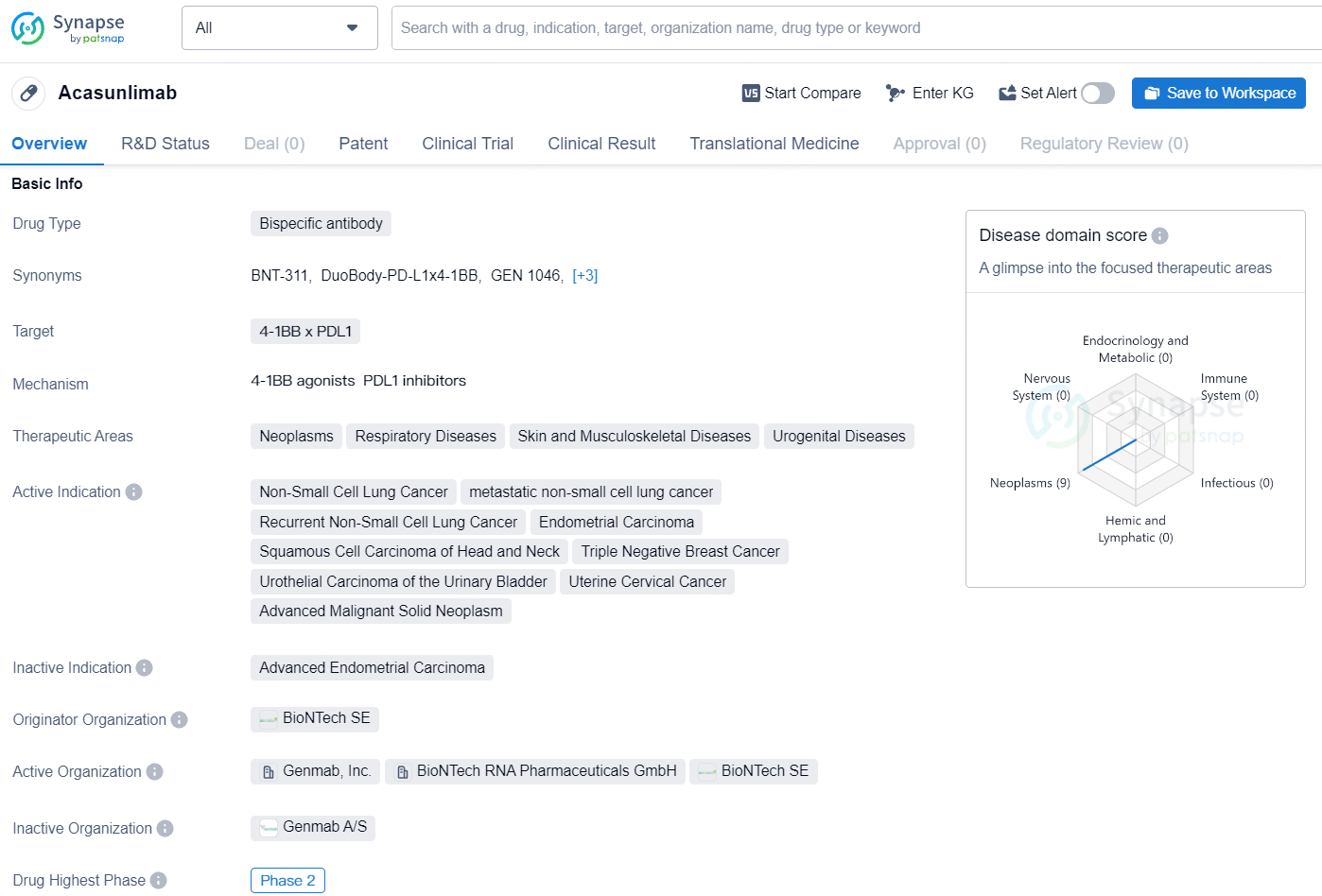
Acasunlimab (GEN1046) is an experimental PD-L1x4-1BB bispecific antibody that harnesses Genmab’s proprietary DuoBody technology platform and BioNTech’s proprietary immunomodulatory antibodies. Designed to provoke an antitumor response, acasunlimab conditionally activates 4-1BB on T cells and natural killer cells, requiring concurrent engagement of the PD-L1 arm.
This therapeutic candidate is currently being evaluated in three clinical studies: (1) a Phase 1/2 trial assessing safety and pharmacokinetics in patients with various solid tumors, (2) a Phase 1 dose escalation study in patients with advanced solid tumors in Japan, and (3) a randomized Phase 2 trial evaluating the safety and efficacy of acasunlimab as a monotherapy and in combination with pembrolizumab in patients with non-small cell lung cancer who have not succeeded with prior immune checkpoint inhibitor treatments.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
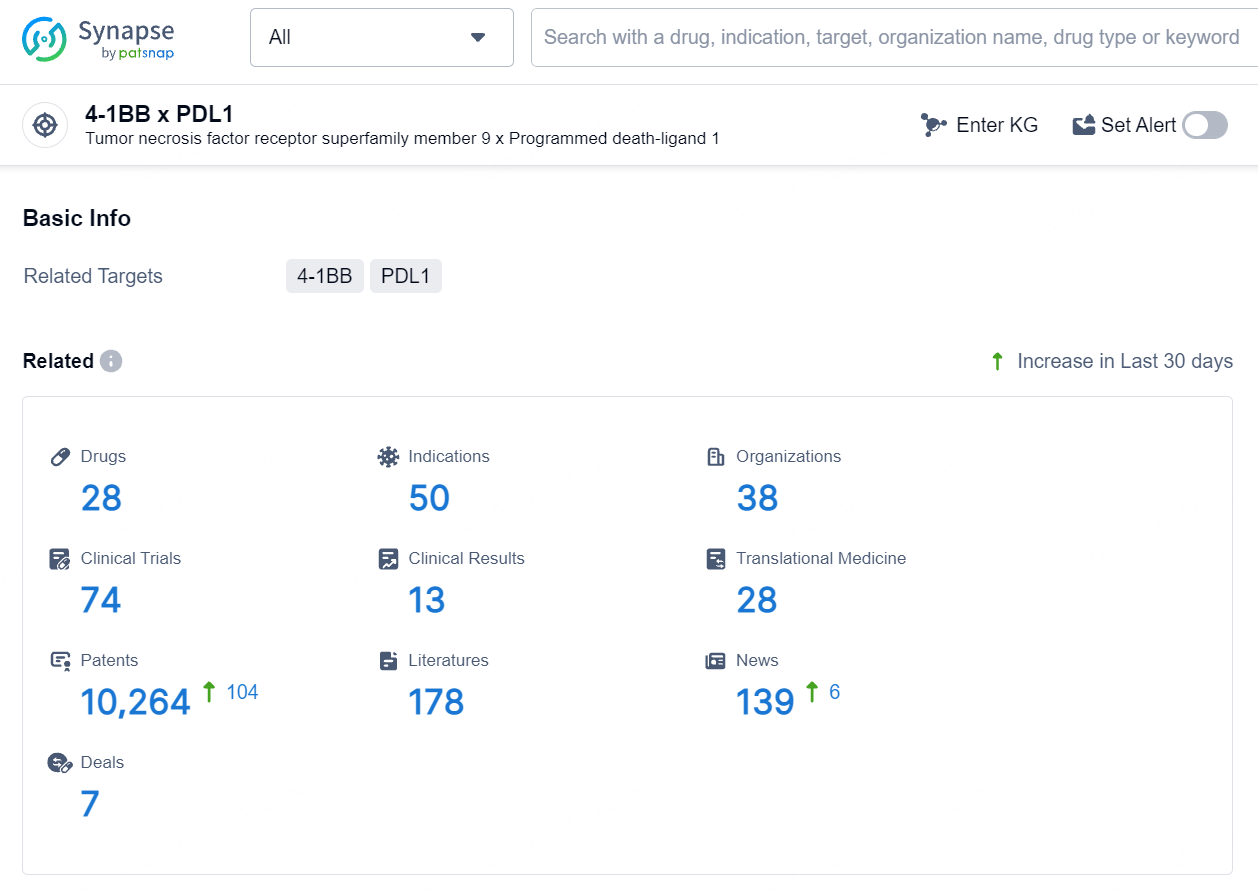
According to the data provided by the Synapse Database, As of August 8, 2024, there are 28 investigational drugs for the PD-L1 and 4-1BB targets, including 50 indications, 38 R&D institutions involved, with related clinical trials reaching 74, and as many as 10264 patents.
Acasunlimab is a bispecific antibody drug that targets the 4-1BB x PDL1 molecules. Acasunlimab represents a promising development in the field of biomedicine, particularly in the treatment of neoplastic and related diseases. As it progresses through clinical trials, its potential to address a wide range of therapeutic areas will be closely monitored within the pharmaceutical industry.