Hanmi Launches ‘HM17321’: New Treatment for Obesity and Muscle Loss Linked to GLP-1
Hanmi Pharm. Co., Ltd. has reported encouraging findings regarding its innovative obesity medication, HM17321. This cutting-edge treatment not only enhances fat reduction but also notably boosts lean muscle mass, positioning it as a potential revolution in the field of obesity treatment. HM17321 tackles a major drawback associated with existing GLP-1-based therapies, which frequently result in muscle loss as an adverse effect of substantial weight loss.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
In addition to HM17321, Hanmi introduced a distinct study focusing on the synergistic effects of HM17321 when used with HM15275, an advanced anti-obesity medication (LA-GLP/GIP/GCG), and semaglutide. The results from this combination therapy revealed a significant decrease in body weight and fat mass compared to the outcomes from individual treatments, effectively mitigating the muscle loss typically associated with extensive weight reduction therapies.
These results highlight HM17321's contribution to enhancing the weight loss experience by promoting fat reduction, boosting muscle mass, and improving muscle functionality, thereby offering benefits in both standalone and combination treatment approaches.
Hanmi also shared additional nonclinical findings related to HM15275 (LA-GLP-1/GIP/GCG), which were initially presented at the American Diabetes Association (ADA) conference. This innovative anti-obesity candidate reportedly has the potential to achieve over 25% weight loss with minimal muscle reduction while providing therapeutic advantages for metabolic conditions. During the 2024 ObesityWeek, Hanmi emphasized HM15275’s mechanism, which supports beneficial metabolic changes and improves energy metabolism when coupled with diet management. These attributes distinctly position HM15275 apart from existing incretin-based obesity therapies that mainly focus on reducing appetite.
Currently, HM15275 is undergoing Phase 1 clinical trials in the United States, with aspirations to advance to Phase 2 trials by 2025, establishing it as a leading candidate in obesity management.
Commenting on these groundbreaking research and development milestones, Dr. Choi remarked, “In this year, Hanmi has solidified its position at the forefront of obesity management with the introduction of HM17321 and HM15275. These advancements continue the progress initiated by efpeglenatide in our H.O.P project. Our dedication to leading-edge R&D will catalyze remarkable progress in the fight against obesity, tackling challenges that were previously seen as unattainable.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
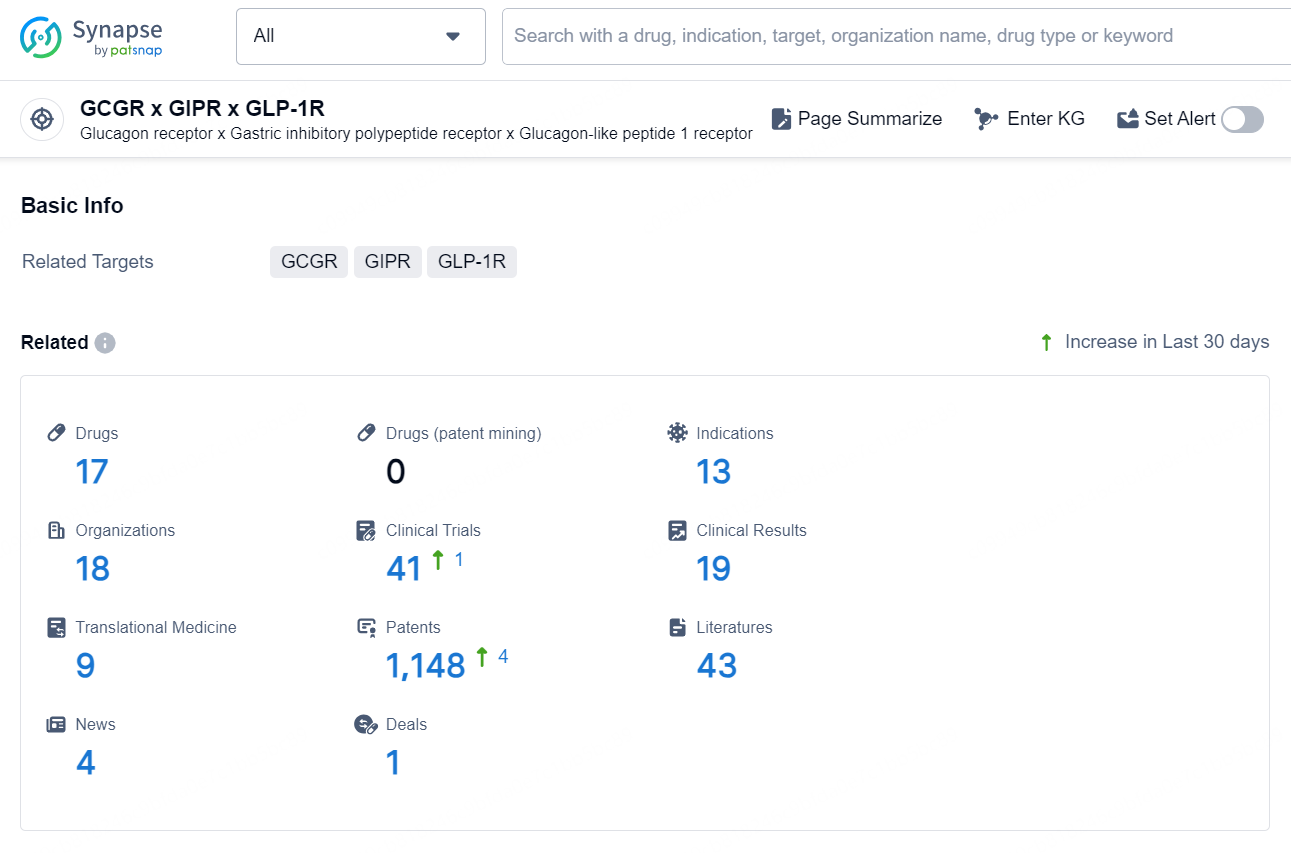
According to the data provided by the Synapse Database, As of November 7, 2024, there are 17 investigational drugs for the GCGR x GIPR x GLP-1R target, including 13 indications, 18 R&D institutions involved, with related clinical trial reaching 41, and as many as 1148 patents.
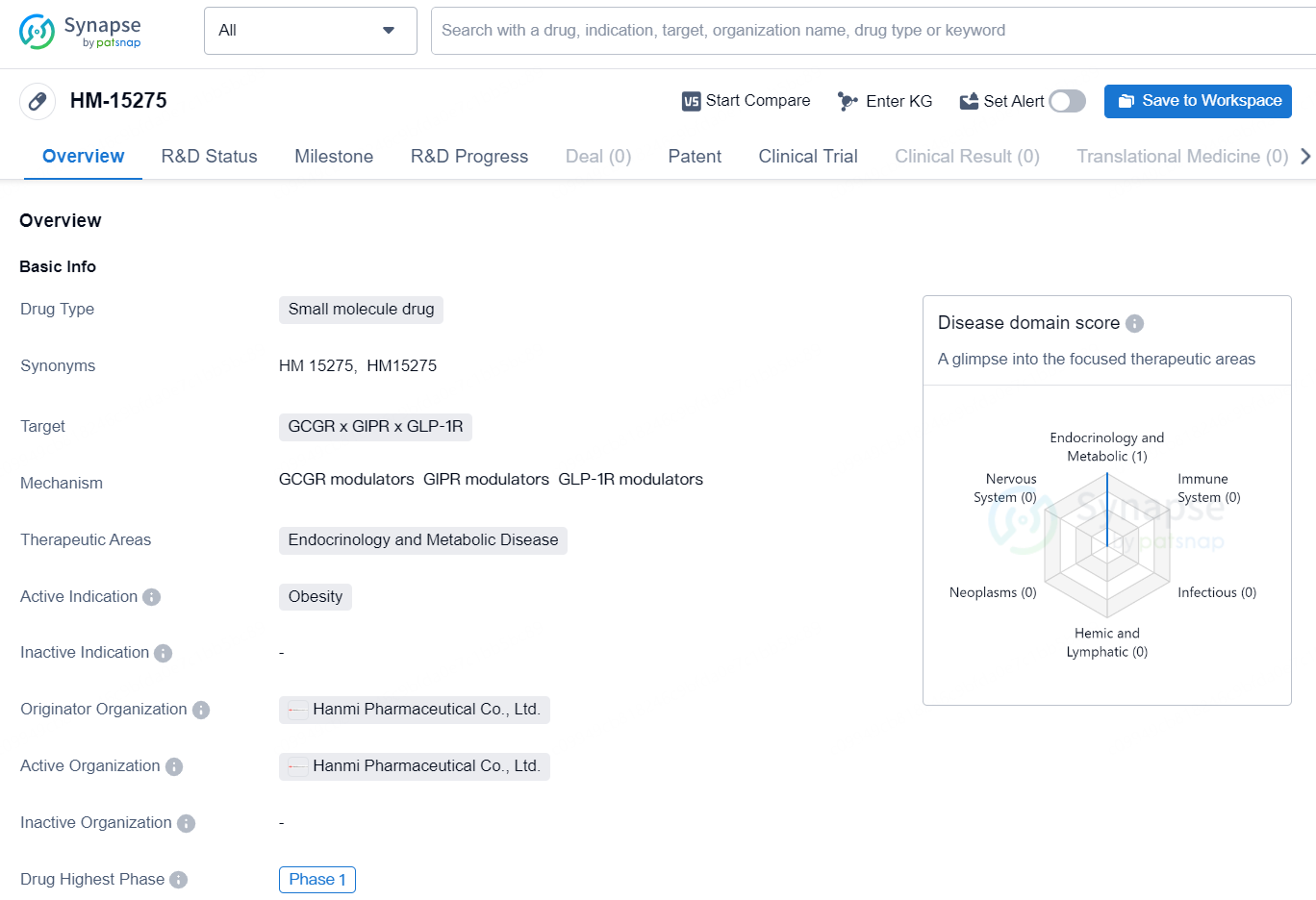
HM-15275 is a small molecule drug that targets GCGR x GIPR x GLP-1R and is being developed for the treatment of obesity in the therapeutic areas of endocrinology and metabolic disease. The drug is in the highest phase of development (Phase 1) globally and is being developed by Hanmi Pharmaceutical Co., Ltd., a pharmaceutical organization with a focus on innovative research and development in the biomedicine field.