Minghui Pharmaceutical Announces Promising Phase I Results for New Bispecific Antibody MHB039A Targeting PD-1 and VEGF
Minghui Pharmaceutical, Inc., a biopharmaceutical firm in the late-stage of development, is dedicated to creating innovative treatments within the fields of immunology and oncology. The company has disclosed preliminary findings from its Phase I clinical trial investigating MHB039A, a new bispecific antibody targeting PD-1 x VEGF, in individuals with relapsed or refractory solid tumors. The study's primary goals are to assess safety and identify the recommended Phase 2 dose (RP2D). Furthermore, secondary aims encompass the characterization of pharmacokinetics, pharmacodynamics, and an initial evaluation of antitumor efficacy.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The research encompassed a patient cohort that had undergone extensive prior treatments, with a median of three previous therapy lines. Significant PD-1 receptor engagement and vascular endothelial growth factor (VEGF) biomarker responses were noted at all dosage tiers. The regimen of 20 mg/kg every three weeks exhibited considerable and prolonged inhibition of both PD-1 and VEGF. Tumor volume shrinkage was observed in patients with squamous non-small cell lung cancer (sqNSCLC) and non-squamous NSCLC lacking actionable genomic alterations (AGA) who had earlier been treated with PD-1 inhibitors and chemotherapy. Additionally, NSCLC patients with EGFR mutations who relapsed after third-generation Tyrosine Kinase Inhibitor (TKI) therapy also showed similar benefits.
MHB039A was found to be well tolerated at doses of up to 20 mg/kg, with the maximum tolerated dose (MTD) not being reached, and no dose-limiting toxicities reported. The overall safety profile was aligned with data from previous clinical trials involving the PD-1 x VEGF bispecific antibody.
"We are very encouraged by the preliminary results from this phase I trial," stated Guoqing Cao, Ph.D., CEO of Minghui Pharmaceutical. "MHB039A has shown a beneficial safety profile alongside promising anti-tumor efficacy in patients with relapsed/refractory solid tumors who have been heavily pretreated. As a bispecific antibody targeting PD-1 and VEGF, it brings together two extensive anti-tumor action mechanisms in a single compound. Notably, this bispecific agent offers superior effects beyond mere additive benefits, demonstrating enhanced anti-tumor effectiveness and significantly better safety in recent clinical evaluations, suggesting its potential as a cornerstone for next-generation immunotherapy."
"The phase I dose escalation trial has concluded," Dr. Cao continued. "Considering the overall attributes of MHB039A, it is well-positioned for synergistic development alongside other therapies, such as chemotherapy, antibody-drug conjugates (ADCs), small molecules, vaccines, and T cell engagers. We are eager to seek strategic collaborations to advance this development."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
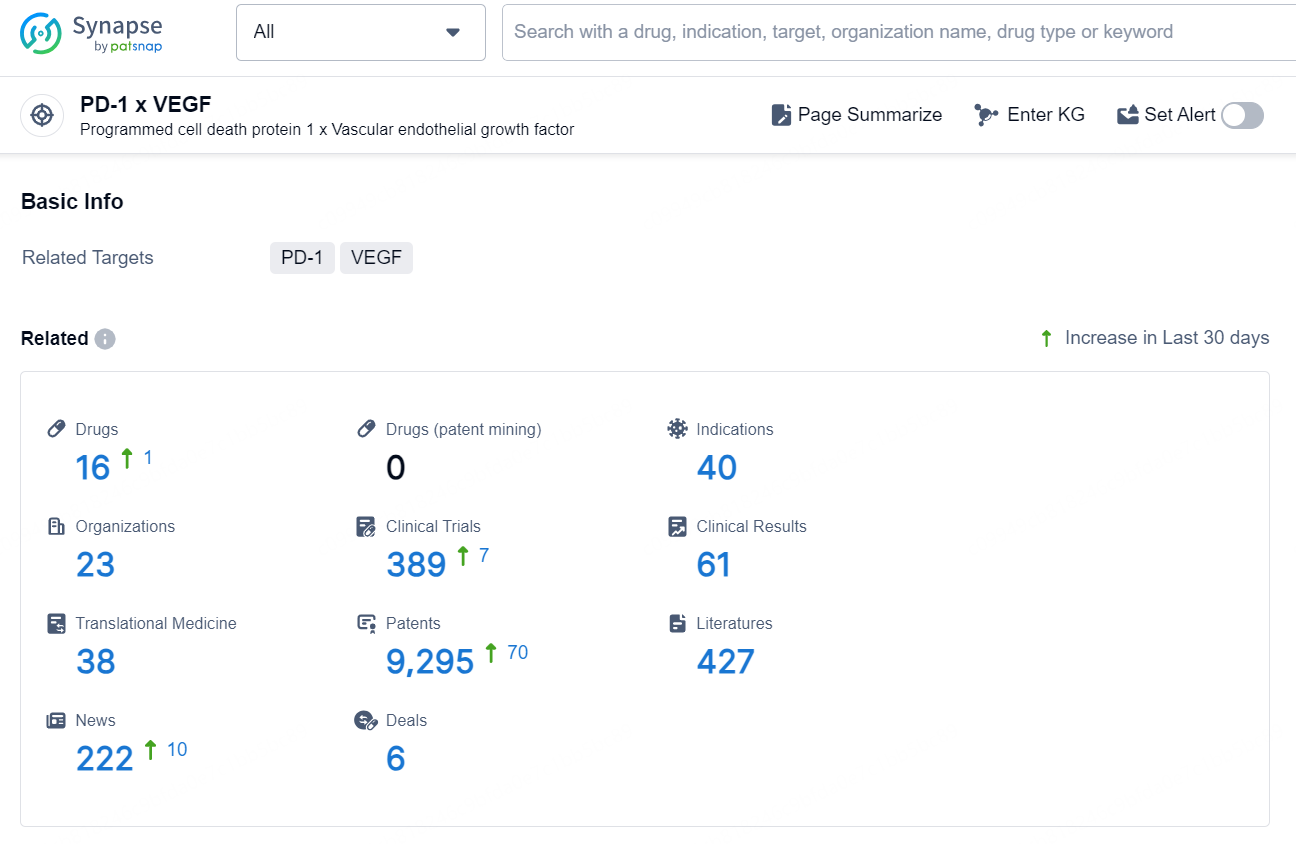
According to the data provided by the Synapse Chemical, As of November 7, 2024, there are 16 investigational drugs for the PD-1 x VEGF target, including 40 indications, 23 R&D institutions involved, with related clinical trial reaching 389, and as many as 9295 patents.
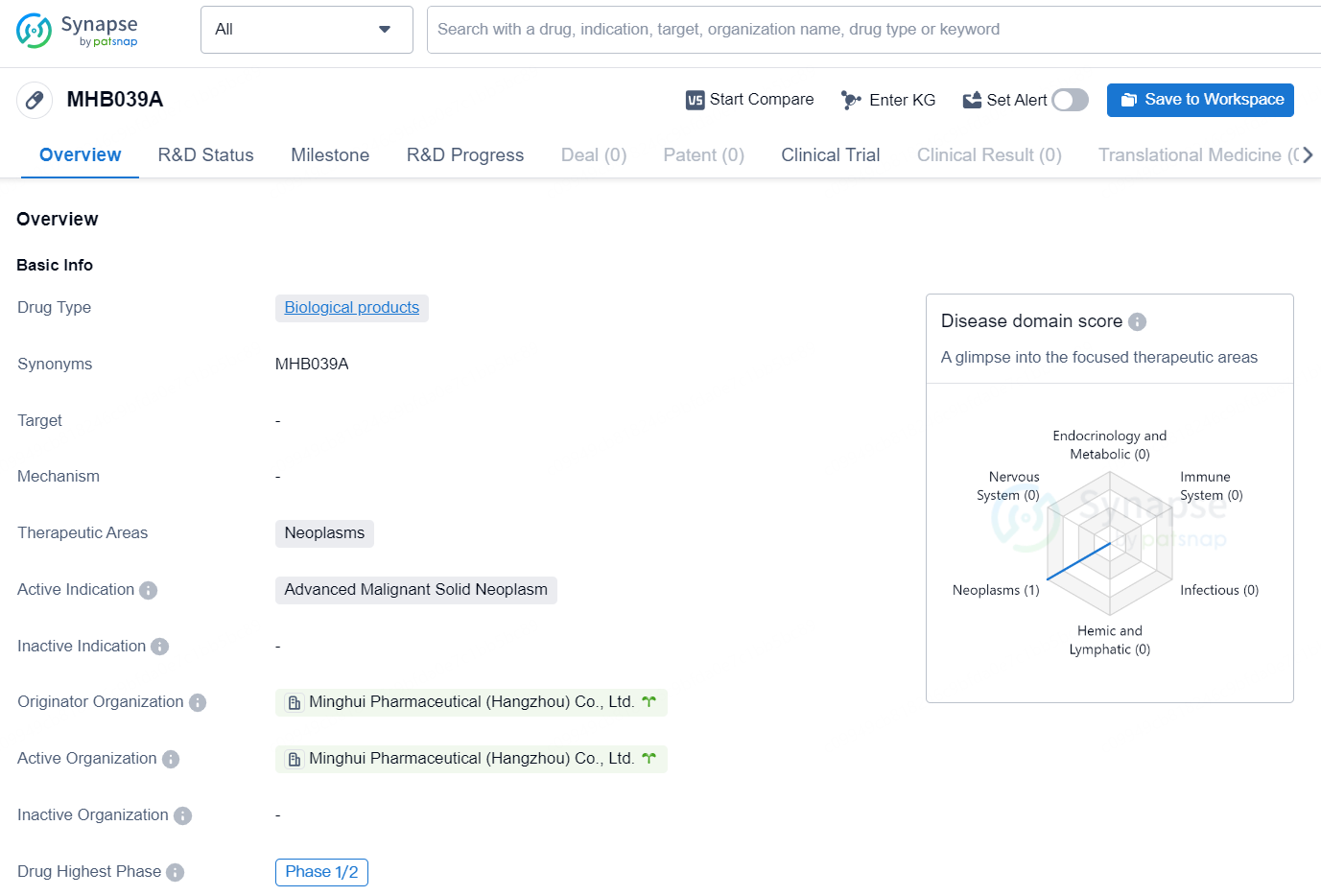
MHB039A is a biological product developed by Minghui Phamaceutical (Hangzhou) CO td.The drug is cumently being developed for the treatment of neoplasms witha specific focus on advanced malignant solid neoplasms The drug has reached Phase 1/2 of clinical development marking an important milestone in its progress towards potential approval and commercialization.