Harbour BioMed Files IND for HBM9378/SKB378 in COPD Treatment
Harbour BioMed is an international biopharmaceutical firm dedicated to the discovery, development, and commercialization of innovative antibody therapies, particularly in the fields of oncology and immunology. The Company recently revealed that it has submitted an Investigational New Drug (IND) application to the Centre for Drug Evaluation within China’s National Medical Products Administration. This application is for HBM9378/SKB378, a fully human antibody designed to target thymic stromal lymphopoietin (TSLP) for the treatment of chronic obstructive pulmonary disease (COPD).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Chronic Obstructive Pulmonary Disease (COPD) is a progressive lung disorder that leads to serious complications such as increased cardiovascular risk, respiratory failure, and lung cancer. The condition represents a significant and escalating burden on healthcare systems and society as a whole. There is an immediate necessity for innovative treatment options that can ease the daily hardships faced by COPD patients and enhance their overall quality of life.
"HBM9378/SKB378 features fully human sequences that present a lower immunogenicity risk and improved bioavailability when compared to other competitors targeting TSLP. Its optimized long half-life and exceptional biophysical characteristics further bolster its advantages in dosing and formulation. We are optimistic about the potential of HBM9378/SKB378 and are convinced that this innovative treatment will fulfill the varied requirements of patients with COPD," stated Dr. Jingsong Wang, the Founder, Chairman, and CEO of Harbour BioMed.
The development of HBM9378/SKB378 is a collaborative effort between Harbour BioMed and Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (6990.HK), with both entities sharing global rights equally for this therapy. HBM9378/SKB378 is a fully human monoclonal antibody targeting TSLP, produced using a platform consisting of two heavy chains and two light chains (H2L2). It works by inhibiting the TSLP-mediated signaling pathway through the blockage of TSLP and its receptor interactions. Its extended half-life and superior biophysical attributes contribute to its favorable dosing profile. Currently, the Company has successfully completed its phase I clinical trial in China under IND status for treating moderate-to-severe asthma.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
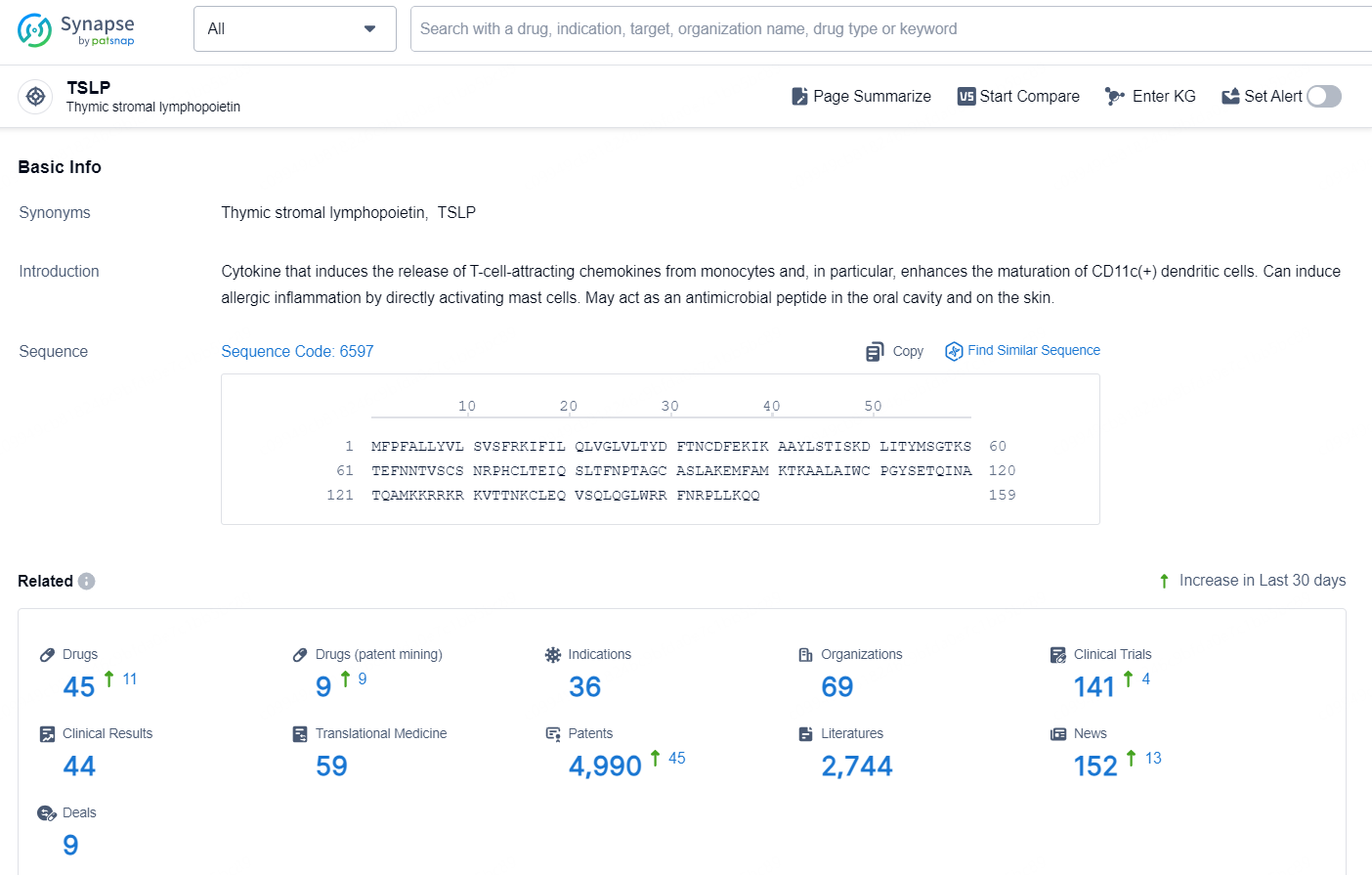
According to the data provided by the Synapse Database, As of November 11, 2024, there are 45 investigational drugs for the TSLP target, including 36 indications, 69 R&D institutions involved, with related clinical trial reaching 141, and as many as 4990 patents.
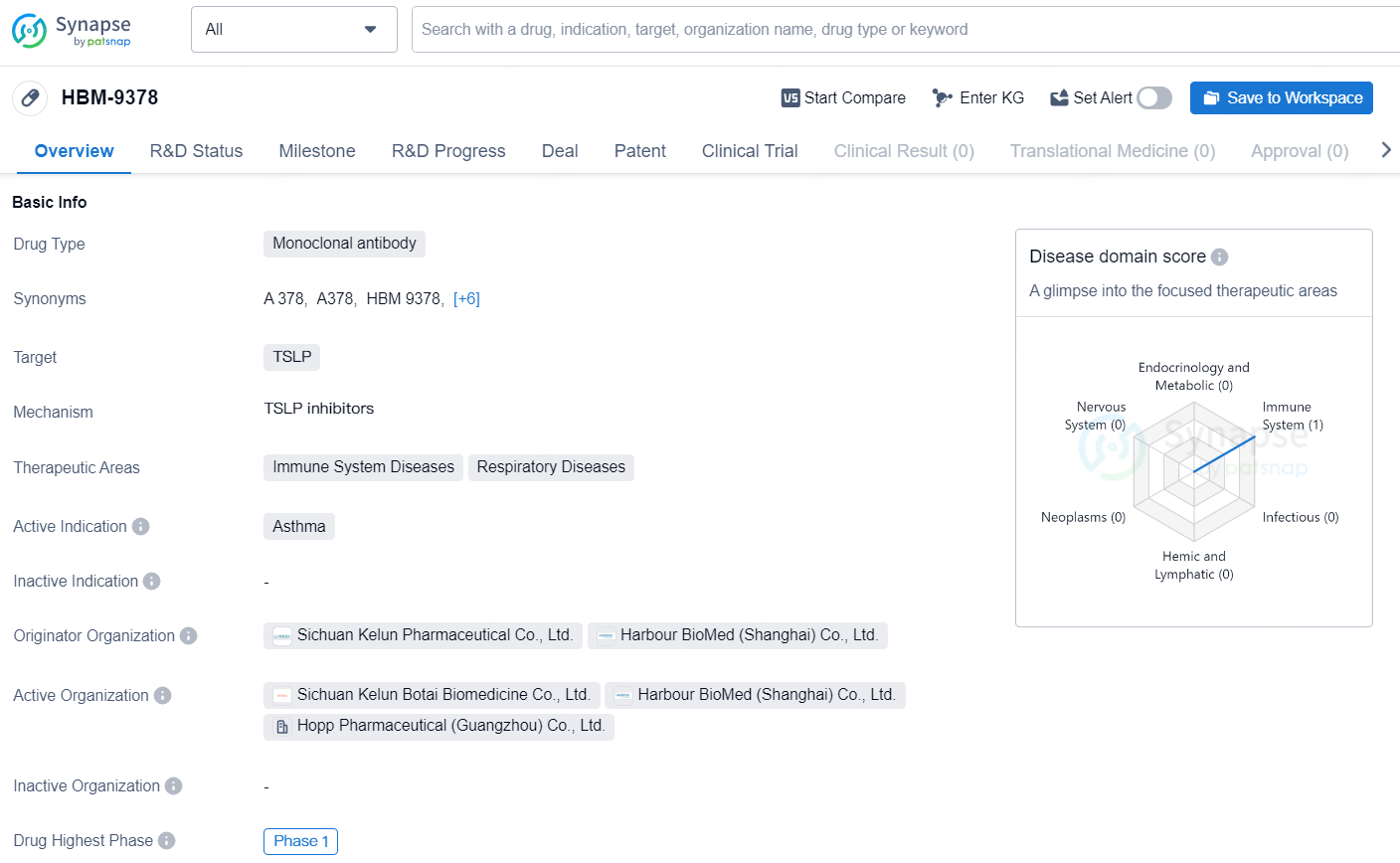
The drug HBM-9378 is a monoclonal antibody designed to target thymic stromal lymphopoietin (TSLP). It is primarily intended for the treatment of immune system diseases and respiratory diseases, with a focus on asthma. The drug has been developed through a collaboration between Sichuan Kelun Pharmaceutical, and Harbour BioMed (Shanghai).