Harnessing Synapse for Triamcinolone Information: Strategies and Tips
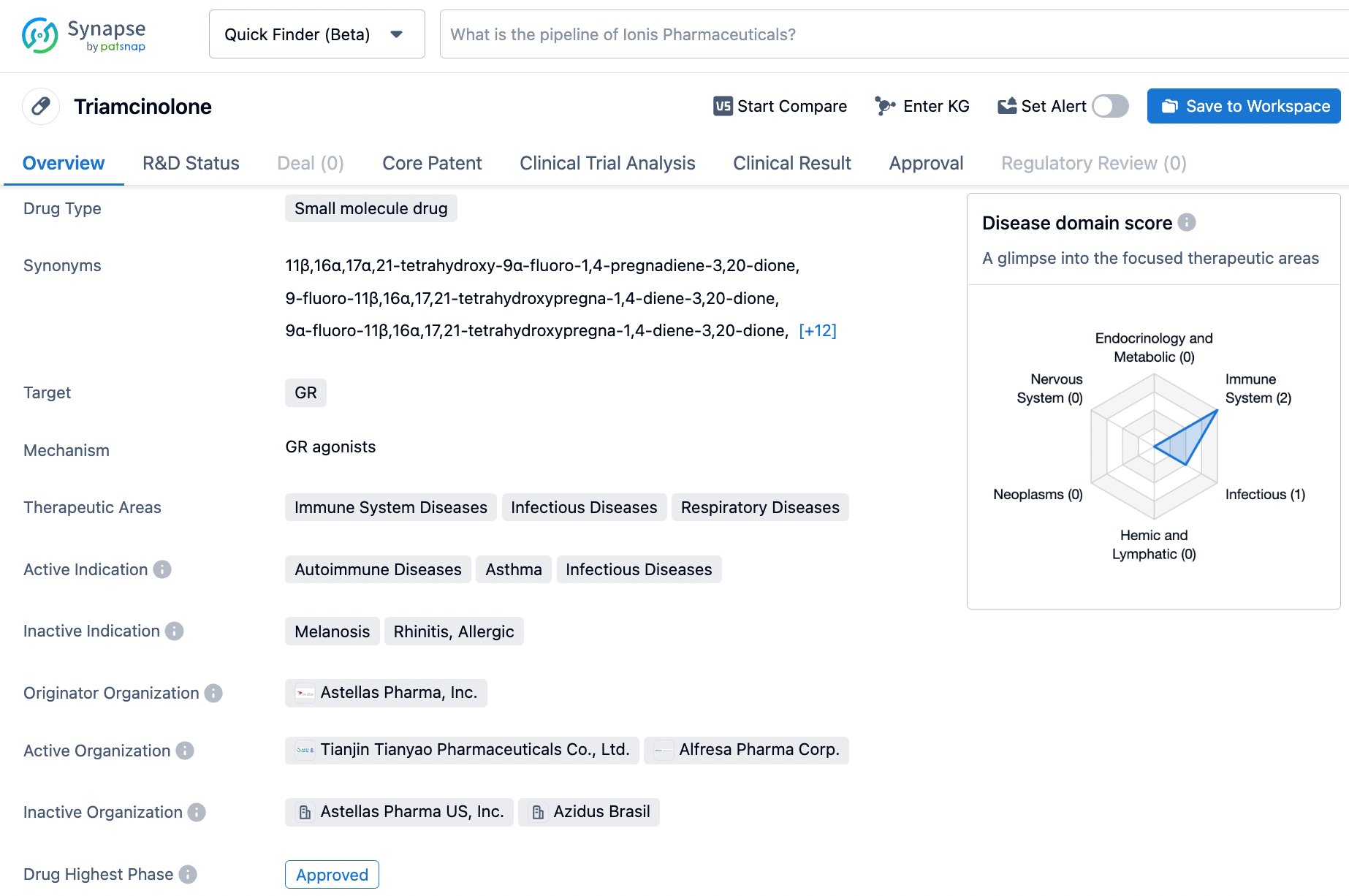
Triamcinolone, an exogenous synthetic corticosteroid, functions as a high-affinity agonist of the glucocorticoid receptor (GR), which is predominantly employed in the treatment of autoimmune disorders encompassing psoriasis, lupus, and rheumatoid arthritis, amongst others. The said chemical entity was developed and brought to market by Astellas Pharma, Inc., having secured regulatory approval for therapeutic use as far back as 1996. Depending on the gravity and location of the condition being managed, triamcinolone can be administered in a multitude of formulations, ranging from injectables to topicals and inhalers. Despite its established therapeutic value in the management of a diverse range of autoimmune disorders, the chemical entity has the potential to evoke an array of unwanted effects, including but not limited to mood changes, weight gain, and fluid retention, especially in a subset of the patient population. Click on the image below to begin the exploration journey of Triamcinolone through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!