Henlius Receives NMPA IND Approval for Phase 1b/2 Study of PD-L1-Targeting ADC HLX43
Shanghai Henlius Biotech, Inc. (2696.HK) has reported that its investigational new drug (IND) application for the phase 1b/2 clinical trial of HLX43 for Injection, an antibody-drug conjugate (ADC) produced in collaboration with MediLink Therapeutics, has received approval from the China National Medical Products Administration (NMPA). This product is intended for use as a monotherapy or in combination therapy for patients with advanced or metastatic solid tumors. In November 2023, the first participant in the phase 1 clinical trial of HLX43 was successfully dosed, marking it as the pioneer PD-L1-targeting ADC in China to enter clinical testing. As of now, there are no PD-L1-targeting ADCs that have been granted marketing authorization worldwide.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Immune checkpoint inhibitors, particularly PD-1/PD-L1 monoclonal antibodies, have gained prominence in recent years, transforming treatment approaches for cancer patients. Despite this advancement, a significant number of patients with positive PD-L1 expression either do not respond to or develop resistance against PD-1/PD-L1-targeted therapies. PD-L1 is expressed across a range of tumor types, such as non-small cell lung cancer (NSCLC), colorectal cancer (CRC), and triple-negative breast cancer (TNBC), while showing minimal expression in normal tissues. This characteristic positions PD-L1 as a viable target for antibody-drug conjugates (ADCs), presenting potential new avenues for cancer therapy.
Currently, there are no subsequent treatment options for patients who are resistant to PD-1/L1 immunotherapy or who did not experience benefits from existing standard treatments, establishing a crucial unmet medical need within this patient group. ADCs and combinations of ADCs with immunotherapies show promise as potential strategies to enhance patient outcomes.
HLX43 represents one of the initial ADC candidates from Henlius to undergo clinical evaluation. It aims to tackle the challenges of resistance or lack of response to PD-1/L1 immunotherapies, addressing the unmet needs of advanced or metastatic patients. By integrating the precision of targeted monoclonal antibodies with a highly effective cytotoxic agent, HLX43 may produce anti-tumor responses through specific binding to PD-L1 on tumor cells, subsequently releasing cytotoxic agents following internalization by these cells. So far, HLX43 has demonstrated considerable anti-tumor activity and a favorable safety profile in preclinical pharmacological, pharmacokinetic, and safety assessments, with findings presented as a poster at the 2023 European Society of Medical Oncology (ESMO) Congress.
Moreover, a phase 1 clinical trial is underway to assess the safety, tolerability, and pharmacokinetics of HLX43 in patients with advanced or metastatic solid tumors. The trial has shown that a 4.0 mg/kg dose of HLX43 administered intravenously every three weeks was well tolerated, with patients suffering from solid tumors—including NSCLC and cervical cancer—showing responses following prior standard treatments. Consequently, Henlius intends to initiate a phase 1b/2 clinical trial to further investigate the efficacy and safety of HLX43, both as a standalone treatment and in combination with other therapies, in various solid tumors among patients who have not responded to standard treatments, thereby validating the product's safety and effectiveness across a wider patient demographic.
In alignment with its commitment to meet unmet medical needs, Henlius will continue to advance its innovative portfolio, leveraging the company’s competitive advantage in an integrated antibody drug development platform to provide more high-quality, affordable treatment options for patients around the globe.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
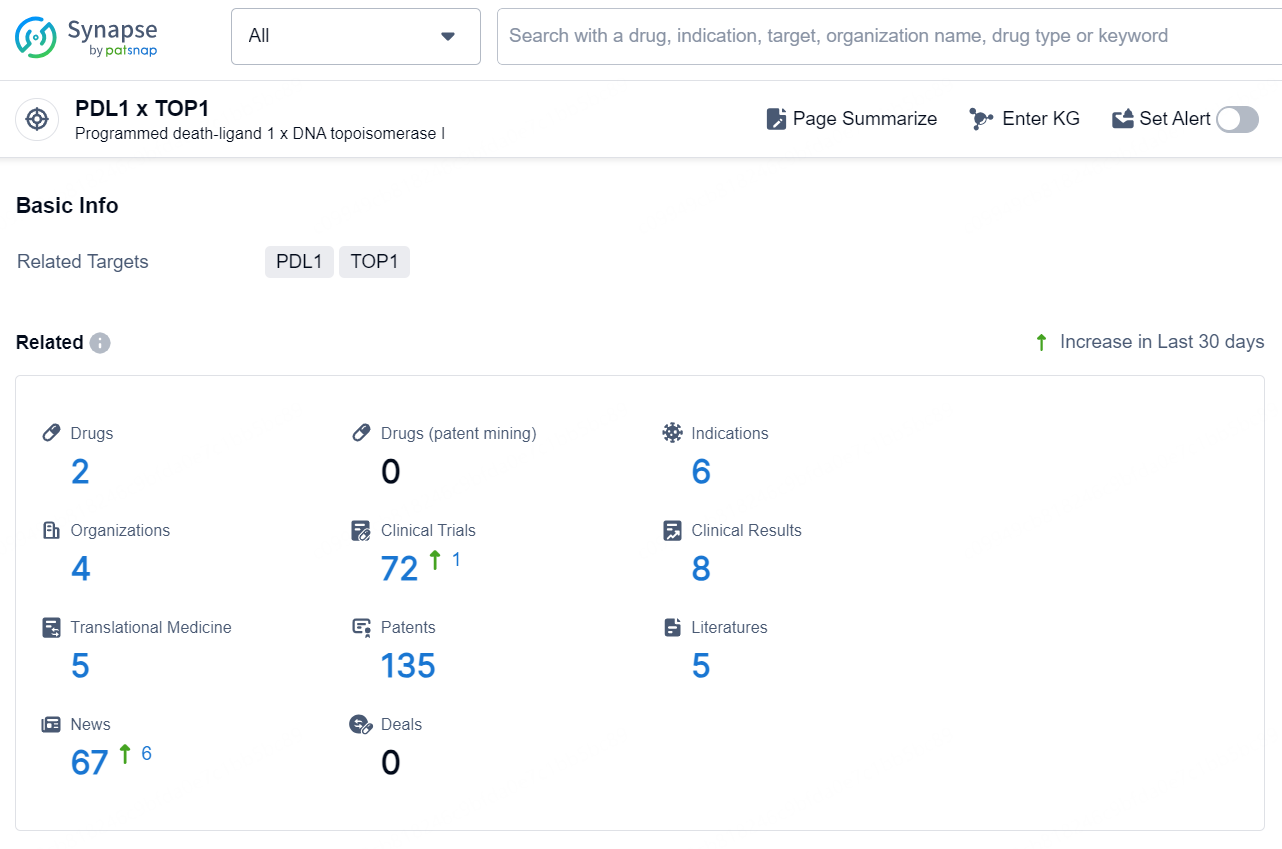
According to the data provided by the Synapse Database, As of December 10, 2024, there are 2 investigational drugs for the PDL1 x TOP1 target, including 6 indications, 4 R&D institutions involved, with related clinical trials reaching 72, and as many as 135 patents.
The drug HLX-43 is an antibody drug conjugate (ADC) that targets PDL1 x TOP1. It falls within the therapeutic areas of neoplasms, with active indications for solid tumor, metastatic solid tumor, and advanced malignant solid neoplasm. The drug is developed by Shanghai Henlius Biotech, Inc., and its highest phase of development globally is Phase 1. It has also reached the highest phase of development in China, which is Phase 1.