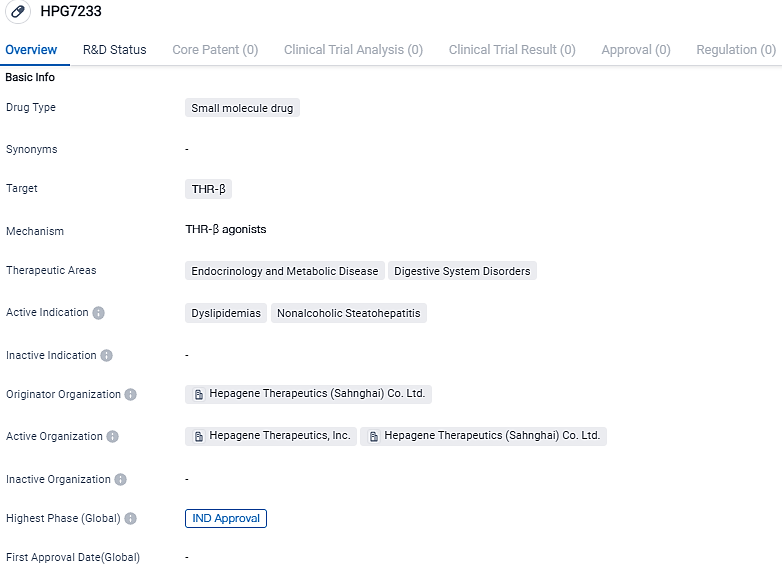
Hepagene Therapeutics Confirms FDA Approval of Experimental New Drug Application (IND) for HPG7233 THR Beta Stimulant
Hepagene Therapeutics, Inc., a firm engaged in clinical-stage biopharmaceutical development, revealed that its IND application forHPG7233 has been approved by the FDA in the United States. HPG7233 functions as an activator of the Thyroid Hormone Receptor beta (THR-β). It is intended for use in the therapy of individuals afflicted with non-alcoholic steatohepatitis and dyslipidemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
HPG7233 is a primarily selective small particle thyroid hormone receptor beta activator that is highly prevalent in the liver. In various laboratory-based studies, HPG7233 showed high degrees of selectivity and efficacy, along with a considerable decrease in liver cholesterol and serum LDL-C in suitable animal trials. Importantly, a substantial collaborative effect was seen in the combination sets, predominantly when mixed with HPG1860.
Within relative organ preferences, HPG7233 has showcased a desirable safety and tolerance level in toxicological evaluations, without discernable unfavorable findings linked to THR-α forms or the gastrointestinal system. These findings strongly support sustained advancements in the clinical progression of HPG7233.
"We are thrilled that the FDA has approved the IND application for HPG7233, thereby expanding our NASH pipeline and setting a significant benchmark for Hepagene." expressed Michael X. Xu, PhD, the President and Chief Executive Officer of Hepagene.
Michael X. Xu added further, "We anticipate starting the clinical study on HPG7233 shortly and eagerly await the successful realization of this project. To augment the moderate effectiveness observed with the initial series of NASH contenders, we will concurrently delve into investigative studies of the THR-β activator in combination with internal FXR and/or GLP-1R activators." Considering its superior preclinical benchmarks, HPG7233 has immense potential to evolve as the upcoming generation of THR-β activators to benefit patients suffering from NASH and dyslipidemia..
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
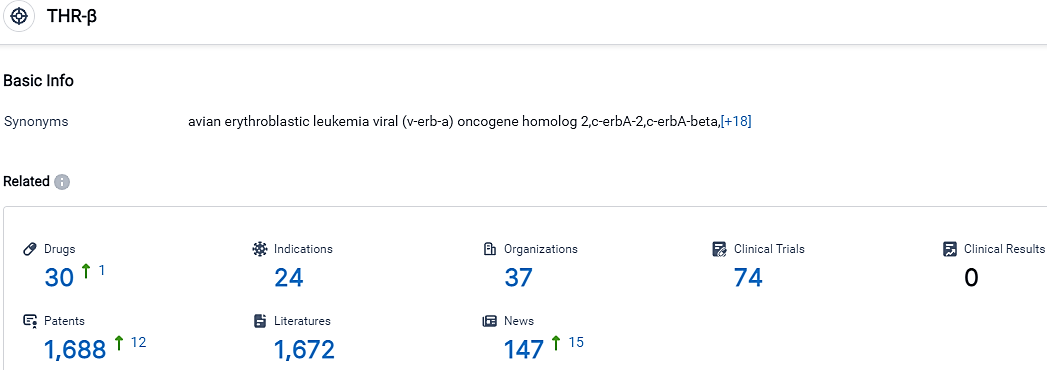
According to the data provided by the Synapse Database, As of September 26, 2023, there are 30 investigational drugs for the THR-β target, including 24 indications,37 R&D institutions involved, with related clinical trials reaching 74,and as many as 1688 patents.
HPG7233 emerges as a possible treatment choice for nonalcoholic steatohepatitis and dyslipidemias. Nonetheless, to thoroughly assess its utility in managing these ailments, additional data on its precise mode of action, clinical trial findings, and possible adverse reactions are essential.