Trofinetide: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Trofinetide's R&D Progress
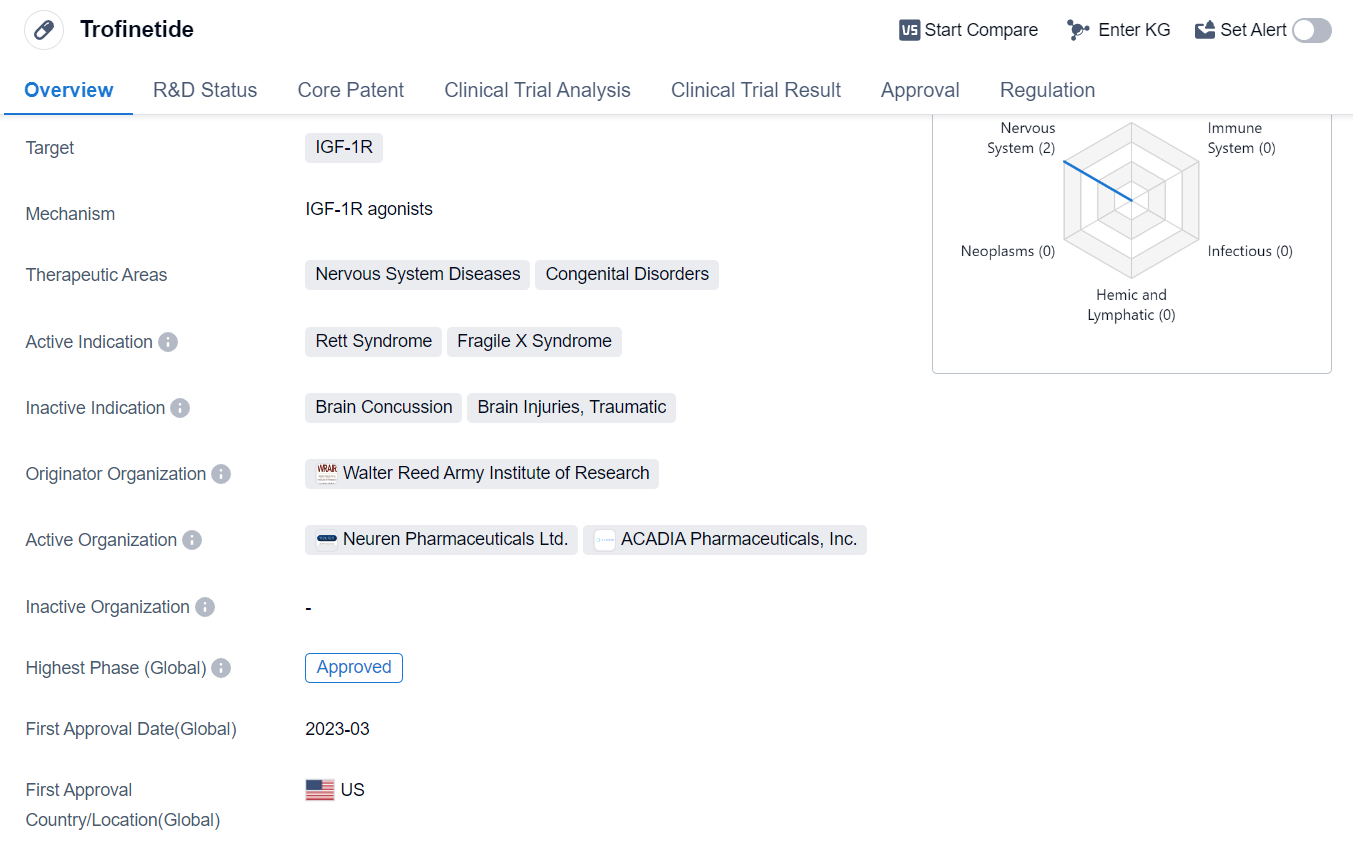
Trofinetide is a synthetic peptide drug that targets the IGF-1R (Insulin-like Growth Factor-1 Receptor). It is primarily used in the treatment of nervous system diseases and congenital disorders. The active indications for Trofinetide include Rett Syndrome and Fragile X Syndrome.
The drug was developed by the Walter Reed Army Institute of Research, an organization known for its contributions to biomedical research. Trofinetide has successfully completed the highest phase of clinical trials and has received approval for use.
Trofinetide received its first approval in the United States in March 2023. The drug has been granted several regulatory designations, including Orphan Drug status, Priority Review, Rare Pediatric Disease designation, and Fast Track designation. These designations highlight the potential benefits of Trofinetide in treating rare diseases and emphasize the need for expedited review and approval processes.
Rett Syndrome is a rare genetic disorder that primarily affects females and leads to severe cognitive and physical impairments. Fragile X Syndrome, on the other hand, is a genetic condition that causes intellectual disabilities and behavioral challenges. Both of these conditions have limited treatment options, making the approval of Trofinetide a significant development in the field of biomedicine.
As a synthetic peptide, Trofinetide is designed to specifically target the IGF-1R, which plays a crucial role in the growth and development of nerve cells. By targeting this receptor, Trofinetide aims to address the underlying mechanisms of Rett Syndrome and Fragile X Syndrome, potentially improving the symptoms and quality of life for patients.
The approval of Trofinetide in the United States and its regulatory designations demonstrate the recognition of its potential therapeutic benefits. The drug's development by the Walter Reed Army Institute of Research further highlights the importance of collaboration between academia and the pharmaceutical industry in advancing medical treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Trofinetide: IGF-1R agonist
IGF-1R agonists are a type of medication or therapeutic agents that specifically target and activate the insulin-like growth factor 1 receptor (IGF-1R). The IGF-1R is a cell surface receptor that plays a crucial role in regulating cell growth, proliferation, and survival. By binding to and activating the IGF-1R, agonists can stimulate various cellular signaling pathways, leading to increased cell growth and proliferation.
From a biomedical perspective, IGF-1R agonists are of significant interest in the field of biomedicine, particularly in cancer research. The IGF-1R signaling pathway is often dysregulated in cancer cells, leading to uncontrolled cell growth and tumor progression. By using IGF-1R agonists, researchers aim to selectively activate the receptor and potentially inhibit tumor growth or sensitize cancer cells to other treatments.
IGF-1R agonists can be developed as therapeutic drugs to target specific diseases or conditions where modulating the IGF-1R signaling pathway is beneficial. However, it is important to note that the development and clinical use of IGF-1R agonists are still under investigation, and their efficacy and safety profiles are being evaluated through preclinical and clinical trials.
Drug Target R&D Trends for Trofinetide
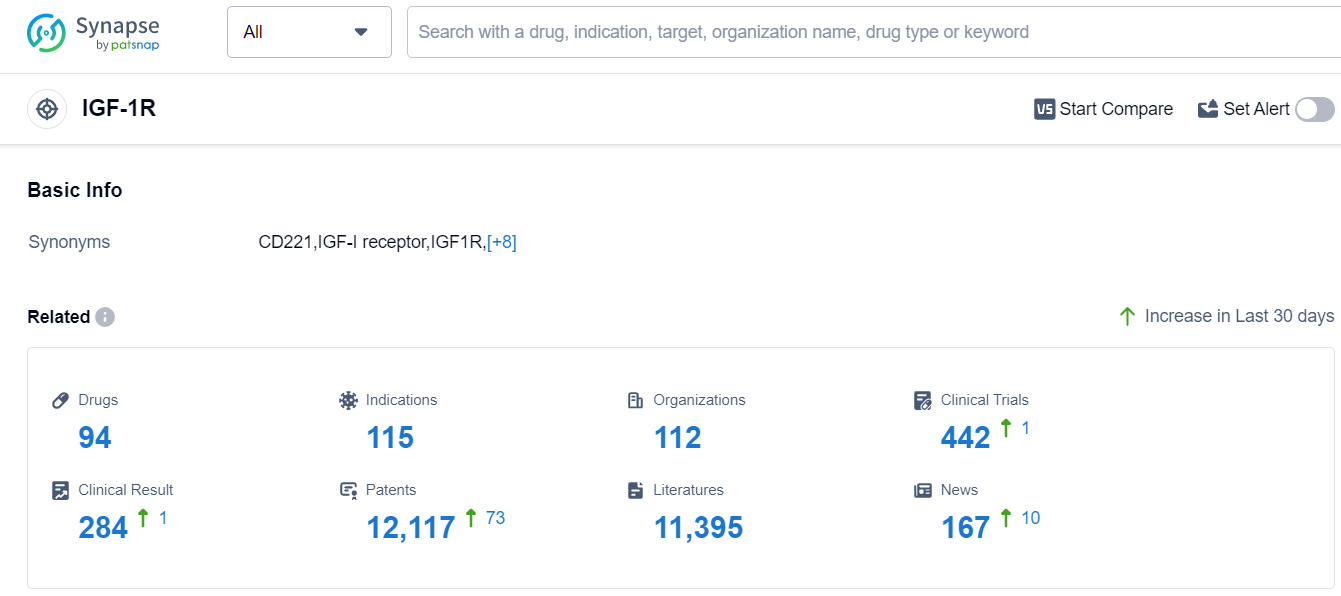
The analysis of target IGF-1R reveals a competitive landscape with several companies showing significant growth and R&D progress. Novartis AG, Takeda Pharmaceutical Co., Ltd., Horizon Therapeutics Plc, and Astellas Pharma, Inc. are among the fastest-growing companies. Approved indications for drugs targeting IGF-1R cover a wide range of diseases, indicating the potential for therapeutic applications. Growth factors, small molecule drugs, and monoclonal antibodies are the most rapidly progressing drug types. The United States, European Union, and Japan are leading in terms of drug development, with China also making progress. Overall, the target IGF-1R presents a promising landscape for the pharmaceutical industry, with potential for further growth and development.
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 94 IGF-1R drugs worldwide, from 112 organizations, covering 115 indications, and conducting 442 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Trofinetide is a synthetic peptide drug that targets the IGF-1R and is approved for the treatment of Rett Syndrome and Fragile X Syndrome. Developed by the Walter Reed Army Institute of Research, Trofinetide has received regulatory designations such as Orphan Drug, Priority Review, Rare Pediatric Disease, and Fast Track. Its approval in the United States marks a significant advancement in the treatment options for these rare genetic disorders.