The Application of CAR-T in Multiple Myeloma
The conventional treatment for multiple myeloma comprises three main pillars: immunomodulatory drugs (thalidomide, lenalidomide, pomalidomide), proteasome inhibitors (bortezomib, ixazomib, carfilzomib), and monoclonal antibodies (daratumumab, isatuximab, elotuzumab, etc.). Additionally, there are several new drugs undergoing research, such as bispecific antibodies, antibody-drug conjugates (ADCs), Selective Inhibitors of Nuclear Export (XPO1), etc. Currently, CAR-T therapy has promising potential in the field of multiple myeloma treatment. CAR-T, short for Chimeric Antigen Receptor T-cell therapy, is not a drug per se, but rather an immunology-based treatment.
Every person naturally possesses two immune mechanisms, or immune defenses. The first line of defense is the innate immune system, a non-antigen-specific natural defense function formed during long-term evolution and individual development. It consists of macrophages, dendritic cells, γδ T cells, NK cells, complements, cytokines, and more within the body. The second line of defense is the adaptive immune system, which includes T cells (also known as cell-mediated immunity) and B cells (also known as humoral immunity). This adaptive immune response is the whole process, during which specific T and B cells become activated, proliferate, differentiate, and induce apoptosis upon antigen stimulation, exhibiting certain biological effects. T cells and B cells can not only eliminate and clear foreign invaders and malignant lesions but also possess a memory function, which activates automatically upon recognizing previous abnormal conditions and carries out immune functions.
CAR-T therapy leverages the immune function of human T cells. The T cells from the patient are collected and subjected to gene editing and amplification outside of the body, enabling the T cells to actively recognize and eliminate malignant tumor cells. Then, the modified T cells are reinfused into the patient to function effectively.
Currently, CAR-T is primarily used to treat malignant hematologic diseases, including refractory multiple myeloma that have been resistant to several lines of treatment. Concurrently, many studies are also exploring its use in the treatment of solid tumors. Clinical data from various studies indicate that CAR-T has shown promising results in treating multiple myeloma, with a notable objective response rate (ORR), complete remission rate (sCR/CR), and progression-free survival (PFS). However, at present, CAR-T can only alleviate conditions and has not reached curative effects yet.
CAR-T therapy also has risks. The most common complication is Cytokine Release Syndrome (CRS), it occurs due to the rapid expansion of CAR-T cells in a short period, leading to massive cytokine release causing symptoms such as fever, low blood pressure, and even organ failure. Other side effects include Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), cytopenia, hypogammaglobulinemia, and susceptibility to infections. Despite this, the aforementioned side effects are generally controllable.
In terms of treating multiple myeloma, the National Medical Products Administration (NMPA) in China has approved a BCMA-targeted CAR-T product for marketing. Currently, there are over 500 CAR-T research projects worldwide, mainly covered by China and the US. Key challenges for CAR-T therapies, apart from side effects, include high costs - the high price of over 1.2 million RMB per injection deters most individuals. This high cost is due to the lengthy period of 20-30 days required to prepare "custom-made" therapy. Limitations such as CAR-T off-target effects, T cell exhaustion, and tumor cell gene mutations also lead to relapse in some patients. Additionally, the follow-up period after CAR-T treatment is not long enough, requiring further support from large sample data over a more extended period.
To address these issues, researchers are developing off-the-shelf CAR-T therapies that involve the collection of T-cells from healthy individuals. This also significantly reduces the cost while continuing to explore new therapeutic targets on the basis of CD19, BCMA, and others. Apart from known CAR-T therapies, autologous hematopoietic stem cell transplantation + CAR-T, TCR-NK, TCR-T, CAR-NK, CAR-M etc., are also current hot research topics. The emergence of immunotherapy suggests a paradigm shift in cancer treatment - from pursuing more potent drug efficacy and more precise targets towards harnessing the body's own immune system to eliminate tumor cells, achieving cure or long-term progression-free survival.
The level of treatment for multiple myeloma will continue to break through
Understanding nature is a lengthy process for humankind. Since the medical community confirmed multiple myeloma in the 1840s, the treatment primarily used cytotoxic drugs for a long time. It wasn't until the end of the last century that immunomodulatory agents such as thalidomide and proteasome inhibitors such as bortezomib came into existence, leading to an overall accelerated development period in the field of multiple myeloma treatment. The efficacy of treatments for myeloma has continuously been enhanced and patient survival has been significantly prolonged, with some scholars even proposing the concept of 'functional cure'. With the rapid advancement of generations of drugs, the addition of monoclones, double clones, antibody-conjugates, and selective nucleocytoplasmic transport inhibitors, myeloma treatment has entered an historical "gush" phase.
We have reason to believe that as hematological research continues to deepen, particularly with the introduction of CAR-T therapy, there will be more and more opportunities for patients to achieve strict complete response (sCR), or MRD negativity post-treatment. Humanity will move closer and closer towards the ultimate goal of curing multiple myeloma.
CAR-T Competitive Landscape
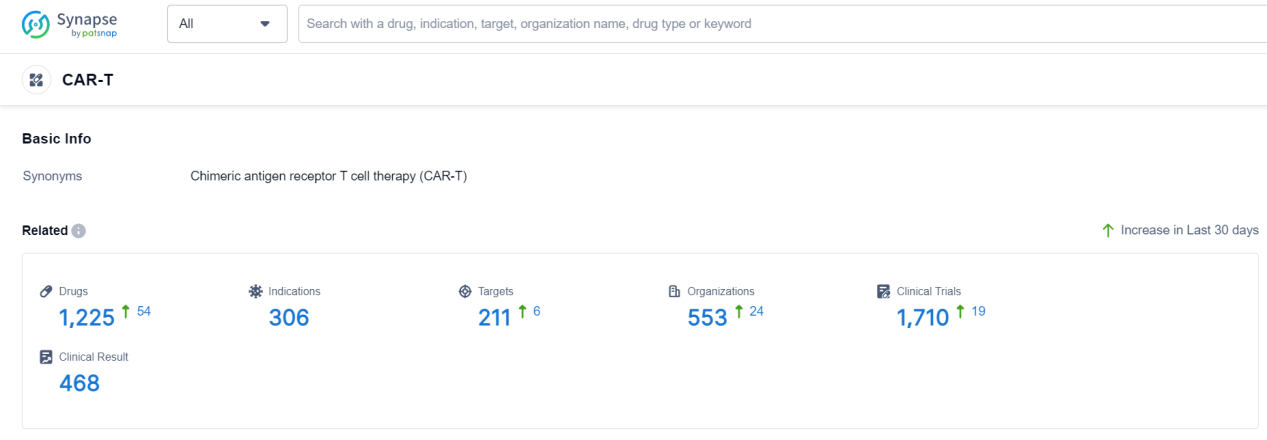
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 1225 CAR-T drugs worldwide, from 553 organizations, covering 211 targets, 306 indications, and conducting 1710 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
Phase I Clinical Trial of CAR-T Medicinal: ATHENA CAR-T
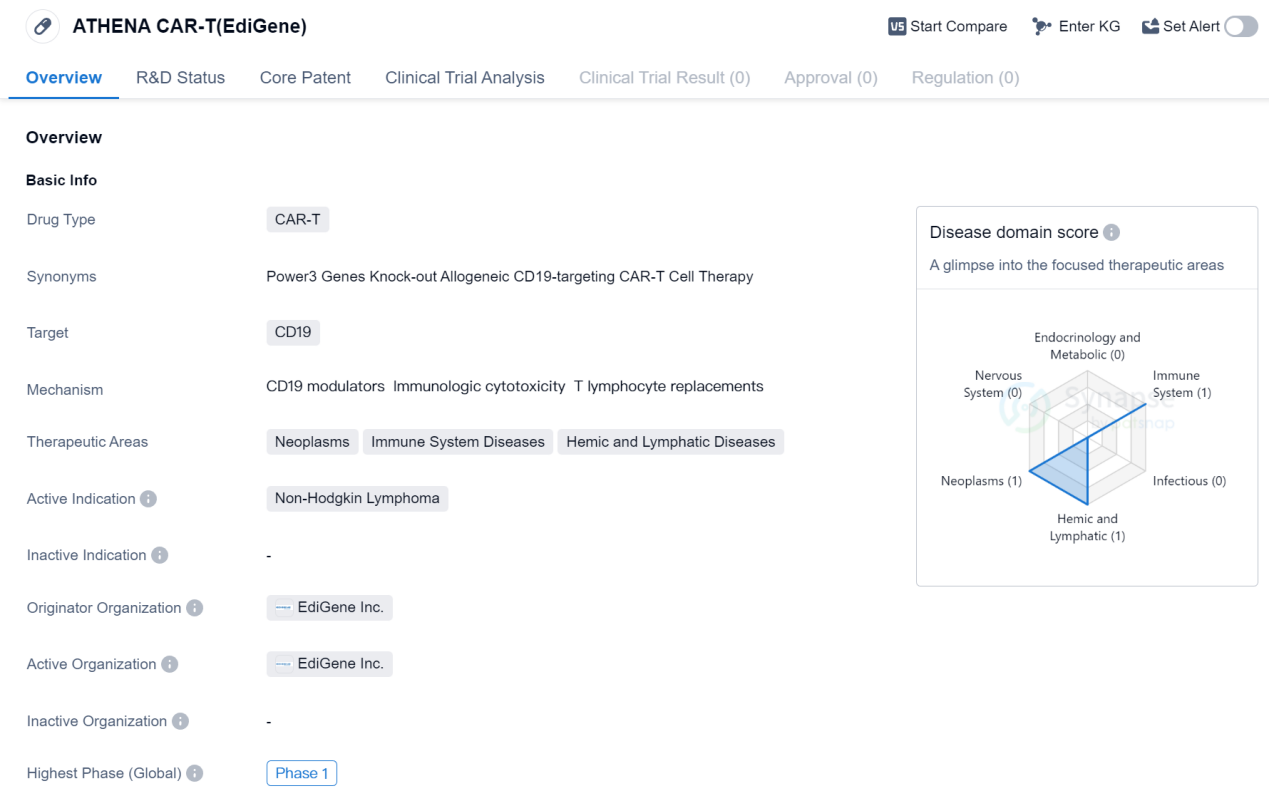
ATHENA CAR-T (EdiGene) is a CAR-T therapy drug that targets CD19, a protein found on the surface of certain cancer cells. It is being developed by EdiGene Inc., a pharmaceutical company specializing in gene editing technologies. The drug is currently in Phase 1 of clinical trials globally.
CAR-T therapy is a type of immunotherapy that involves modifying a patient's own immune cells to recognize and attack cancer cells. CD19 is a common target for CAR-T therapies, as it is expressed on the surface of B cells, which are involved in various types of cancers, including Non-Hodgkin Lymphoma.
Non-Hodgkin Lymphoma is a type of cancer that affects the lymphatic system, which is part of the immune system. It is characterized by the abnormal growth of lymphocytes, a type of white blood cell. The development of ATHENA CAR-T (EdiGene) aims to provide a potential treatment option for patients with Non-Hodgkin Lymphoma.
As the drug is currently in Phase 1 of clinical trials, it is still in the early stages of development. Phase 1 trials primarily focus on evaluating the safety and dosage of the drug in a small group of patients. The results from these trials will help determine the appropriate dosage and potential side effects of ATHENA CAR-T (EdiGene).
The therapeutic areas of ATHENA CAR-T (EdiGene) include neoplasms (cancer), immune system diseases, and hemic and lymphatic diseases. This suggests that the drug may have potential applications beyond Non-Hodgkin Lymphoma, although further research and clinical trials would be needed to explore these possibilities.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Overall, ATHENA CAR-T (EdiGene) shows promise as a CAR-T therapy targeting CD19 for the treatment of Non-Hodgkin Lymphoma. However, as it is still in the early stages of development, more research and clinical trials are needed to determine its efficacy and safety profile.