Highlights of February 2025: Biopharmaceutical Clinical-Stage Drug Investment, Financing, and Collaboration Deals (1)
In February 2025, the global biopharmaceutical industry remains active in clinical-stage drug financing and collaboration activities. For instance, XtalPi Holdings successfully raised over HKD 3.2 billion via two equity placements, which will be used to further develop its AI+CRO business model. Additionally, in the realm of innovative drug partnership deals, CSPC Group’s subsidiary signed an exclusive licensing agreement with Radiance Biopharma for the antibody-drug conjugate (ADC) SYS6005, which includes up to $150 million in potential development and regulatory milestone payments as well as up to $1.075 billion in potential sales milestone payments. Innovent Biologics outlined a global exclusive collaboration and licensing agreement with Roche for the next-generation ADC candidate IBI3009, with potential milestone payments reaching up to $1 billion.
1. Nxera Pharma and Viatris Collaborate to Advance the Development and Commercialization of an S1P1 Receptor Modulator in the Asia-Pacific Region
On February 28, 2025, Nxera Pharma reached a significant agreement with Viatris Inc. and Idorsia Pharmaceuticals Ltd regarding the rights to develop and commercialize Cenerimod in Japan, South Korea, and other Asia-Pacific regions (excluding China). Cenerimod, a novel oral S1P1 receptor modulator, holds significant potential as a first-line or best-in-class therapy for various autoimmune diseases. Under the terms of the agreement, Nxera Pharma will receive an upfront payment of $10 million from Viatris and will be eligible for regulatory milestone payments upon Cenerimod's approval in Japan. Additionally, Nxera Pharma will collect royalties based on net sales within the designated territories.
Cenerimod is a highly selective S1P1 receptor modulator that primarily works by controlling lymphocyte migration to reduce inflammation. Prior to this, Viatris had already secured exclusive global rights to develop and commercialize Cenerimod outside of Japan and the Asia-Pacific region (excluding China) through a 2024 research and development agreement with Idorsia. This new partnership with Nxera Pharma further reinforces Viatris's global positioning in drug development and provides robust support for Cenerimod's ongoing research and development.
The mechanism of Cenerimod lies in its selective targeting of the S1P1 receptor. By inhibiting lymphocyte egress from peripheral lymphoid organs into systemic circulation, Cenerimod effectively suppresses inflammatory responses. This allows it to address the abnormally elevated levels of type I interferons (IFN-1) and other pro-inflammatory cytokines observed in autoimmune diseases such as systemic lupus erythematosus (SLE).
This collaboration underscores the critical role of cross-company partnerships in accelerating the development of innovative medicines. It not only expedites market entry for novel drugs to meet unmet medical needs but also effectively distributes research and development risks, fostering sustainable growth within the pharmaceutical industry. For Cenerimod, this collaboration promises broader market access and technical support, paving the way for potential breakthroughs in the treatment of autoimmune diseases.
2. Shuangqi Pharmaceutical and Anaerobic Biotechnology Collaborate to Advance the Development and Commercialization of a Quadrivalent Lactobacillus Vaginal Live Biotherapeutic Capsule
Inner Mongolia Shuangqi Pharmaceutical has entered into a significant agreement with Anaerobic Biotechnology concerning an innovative live biotherapeutic product, KAL-001, for vaginal use. This microecological therapy aims to restore a healthy vaginal environment by regulating its microbiota, specifically targeting vaginal inflammatory conditions in women of reproductive age. KAL-001 is composed of four specific strains of Lactobacillus, identified after a complex process of screening, isolation, preservation, and evaluation by Anaerobic Biotechnology.
KAL-001 works by replenishing beneficial bacterial flora, i.e., Lactobacillus, in the vagina to restore and maintain a healthy microbial balance. This therapeutic approach differs from traditional antibiotic treatments. Instead of directly killing pathogens, it inhibits the growth of harmful microorganisms by increasing the population of beneficial microbes. Lactobacilli produce metabolic byproducts such as lactic acid, which lower vaginal pH and create an inhospitable environment for pathogenic organisms. Additionally, Lactobacillus may competitively exclude other potential pathogens and enhance the host’s local immune response through immunomodulatory mechanisms.
The collaboration extends beyond product development and includes the transfer of commercialization rights. According to the licensing agreement, Shuangqi Pharmaceutical will pay Anaerobic Biotechnology an upfront fee of RMB 25 million and commit to milestone payments of up to RMB 170 million based on development progress and sales performance, along with royalties derived from net sales. This arrangement reflects the confidence both parties have in the market potential of KAL-001 and highlights their commitment to supporting R&D investments.
3. Sino Biopharmaceutical Collaborates with Sciwind Biosciences to Advance the IL-29 Project; CPX102 Expected to Become a Breakthrough Treatment for RSV
Sino Biopharmaceutical has announced the signing of an exclusive strategic collaboration agreement with Sciwind Biosciences for the development and commercialization of the human interleukin-29 (IL-29, also known as interferon-λ1) project. The deal grants exclusive licensing and cooperation rights in 19 countries, including China (including Hong Kong, Macau, and Taiwan), Brazil, Saudi Arabia, Thailand, and Singapore. The partnership aims to expedite the development of CPX102 and expand its applications in the global market.
CPX102 is an inhalable aerosol solution developed through protein engineering based on IL-29. As a type III interferon, it does not directly kill or inhibit viruses but induces antiviral protein production by activating cell surface receptors, thereby preventing viral replication. Additionally, it can activate immune cells, such as cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, thus enhancing the body's innate immune response against viral infections. This treatment is particularly effective for respiratory syncytial virus (RSV) infections in children, as it directly targets the epithelial cells in the lungs via inhalation, allowing for rapid onset of action and significantly improving the clearance of the virus.
As the world's first aerosolized inhalation medication for RSV treatment, CPX102 offers high patient acceptability, particularly for children. Its local administration results in systemic exposure that is only about 1/100 of the injection dosage, drastically reducing the occurrence of systemic side effects. This not only helps avoid the discomfort associated with injections but also makes the treatment process safer and more comfortable, an especially critical factor for pediatric patients with pulmonary viral infections.
As a broad-spectrum antiviral drug, CPX102 activates the host immune system to clear various respiratory viruses, including RSV, SARS-CoV-2, and influenza viruses, and is not affected by viral mutations. Given the current lack of effective treatments for RSV, particularly in high-incidence countries like China, CPX102 has vast market potential. It is estimated that RSV causes approximately 330 million respiratory infections globally each year, with China being one of the countries with the highest incidence rate.
Currently, CPX102 is undergoing Phase IIb clinical trials in China and is expected to become the first Class 1 innovative drug in the country approved for the nebulized treatment of RSV infections in children. Considering the unmet demand for RSV therapies both in China and globally, along with the promising clinical results of CPX102, this drug has the potential to become a blockbuster product in the respiratory and anti-infective therapeutic area, bringing tremendous market value and social benefits.
4. Ligand Pharmaceuticals Invests $75 Million to Support Castle Creek Biosciences’ D-Fi Gene Therapy
On February 25, Ligand Pharmaceuticals announced the completion of a $75 million royalty financing agreement with Castle Creek Biosciences to fund the Phase III clinical development of D-Fi (FCX-007), a gene therapy for dystrophic epidermolysis bullosa (DEB), through its initial data readouts. Of the $75 million investment, Ligand provided $50 million, while leading a consortium—including Paragon Biosciences, Valor Equity Partners, and new investor XOMA Royalty Corporation—that contributed the remaining $25 million. In return, the investors will receive single-digit percentage royalties on global sales of D-Fi.
D-Fi is an autologous gene-modified cell therapy designed to treat DEB, a rare genetic skin disorder caused by mutations in the COL7A1 gene. This condition results in insufficient production of type VII collagen (COL7), which is critical for anchoring the dermis to the epidermis. To address this, D-Fi uses dermal fibroblasts harvested from the patient, modifies them ex vivo with a self-inactivating (SIN) lentiviral vector encoding the COL7A1 gene to produce COL7 protein, and locally injects the treated cells into chronic wounds. This promotes the formation of anchoring fibrils within the skin. Clinical studies have shown that D-Fi is well-tolerated, with the primary adverse reactions being localized injection site responses.
This collaboration underscores Ligand's commitment to investing in breakthrough treatments that have the potential to transform patients’ lives while also diversifying its income-generating asset portfolio. Matthew Gantz, President and CEO of Castle Creek Biosciences, expressed support for the partnership, emphasizing its significance as a critical step towards addressing the unmet needs of patients with DEB.
5. Eli Lilly Acquires Organovo's FXR Program, Including Key Therapy FXR314
On February 25, 2025, Organovo Holdings announced that Eli Lilly and Company would acquire its FXR program, including its core asset FXR314. This transaction marks a significant milestone for Organovo in advancing drug development for inflammatory bowel disease (IBD) using their proprietary 3D human tissue models.
FXR314 is a potentially "best-in-class" oral farnesoid X receptor (FXR) agonist, initially developed to target metabolic dysfunction-associated steatohepatitis (MASH). However, recognizing the immense market potential of inflammatory bowel disease and the limitations of existing therapeutic options, Organovo deemed FXR314 equally compelling for the IBD space. Preclinical studies have demonstrated that FXR314 improves key indicators of ulcerative colitis in a dose-dependent manner using an adoptive T-cell transfer model.
Under the agreement terms, Organovo will receive an upfront payment from Eli Lilly, in addition to milestone payments as FXR314 progresses through critical regulatory and commercial achievements. Moreover, Eli Lilly will obtain worldwide rights to develop and commercialize Organovo’s FXR program, including all associated intellectual property. Organovo anticipates reporting results from the Phase IIa clinical trial of FXR314 for ulcerative colitis in the first half of 2026.
Through this acquisition, Eli Lilly not only strengthens its portfolio in the inflammatory bowel disease field but also underscores its commitment to innovative therapies. Previously, Eli Lilly successfully launched the IL-23 monoclonal antibody Mirikizumab for treating ulcerative colitis and Crohn’s disease, achieving significant results. With the addition of FXR314 from Organovo, Eli Lilly is well-positioned to further expand its impact in IBD treatment, offering new hope to patients.
6. CARsgen Therapeutics Partners with SoftBank Xinchuang to Accelerate the Development of Off-the-Shelf CAR-T Cell Therapies in China
On February 25, 2025, CARsgen Therapeutics announced a strategic partnership with SoftBank Xinchuang, jointly investing in Ukaize Biopharmaceuticals. This collaboration aims to accelerate the development and commercialization of CARsgen’s two off-the-shelf CAR-T cell therapy products in mainland China. These products include a BCMA CAR-T therapy targeting multiple myeloma and plasma cell leukemia, as well as a CD19/CD20 CAR-T therapy for B-cell malignancies. Through this partnership, CARsgen seeks to leverage external capital to expedite innovation in the CAR-T therapy field.
As part of the agreement, a SoftBank Xinchuang-managed fund will subscribe to RMB 80,000,000 of newly issued registered capital in Ukaize Biopharmaceuticals, acquiring an 8% equity stake upon transaction completion. This financing not only provides essential funds for Ukaize but also reduces CARsgen’s ownership in Ukaize from 100% to 92%. The capital investment will be allocated to support Ukaize’s research, production, and commercialization efforts. For a rapidly evolving biotech company, such funding is vital to advancing clinical trials and shortening the time required to bring new products to market.
CARsgen’s two CAR-T cell therapy products target key molecular markers: BCMA (B-cell maturation antigen) and CD19/CD20. The BCMA CAR-T therapy is primarily designed to treat multiple myeloma and plasma cell leukemia—both of which are challenging, refractory hematologic malignancies. Meanwhile, the CD19/CD20 CAR-T therapy is developed to combat B-cell-derived malignancies. These therapies work by genetically engineering patients' T cells to recognize and destroy cancer cells, offering a new form of personalized treatment.
This approach represents a significant advancement in the field of cancer treatment by enabling precision-targeted therapy against cancer cells while minimizing damage to normal tissues.
7. CSPC Licenses ROR1-Targeting ADC to Radiance Biopharma
On February 19, 2025, CSPC Pharmaceutical Group announced that it has granted Radiance Biopharma exclusive rights for the development and commercialization of its proprietary ROR1-targeting antibody-drug conjugate (ADC), SYS6005, in the United States and other major markets. Under the terms of the agreement, Radiance Biopharma will pay an upfront fee of $15 million, with additional development and regulatory milestone payments of up to $150 million, and sales milestone payments totaling up to $1.075 billion. Furthermore, Radiance Biopharma will pay tiered royalties based on the annual net sales of the product in the licensed territories. This collaboration marks another major milestone for CSPC in its global market expansion.
SYS6005 is a monoclonal antibody-drug conjugate specifically designed to bind to the ROR1 receptor on the surface of tumor cells. Through endocytosis, the ADC delivers the potent mitotic inhibitor MMAE into cancer cells, where it releases its cytotoxic payload to effectively kill the cancer cells. This targeted design enhances therapeutic efficacy while minimizing damage to healthy tissues. SYS6005 received approval from the Chinese National Medical Products Administration (NMPA) to enter clinical trial stages by the end of 2024, with indications including hematological malignancies, ovarian cancer, non-small cell lung cancer, and other advanced malignancies. These developments highlight the broad potential application of SYS6005 in oncology.
8. Brexogen and BMI Korea Enter Technology Transfer Agreement for Exosome-Based Therapy
On February 19, 2025, Brexogen, a biotechnology company specializing in exosome-based therapies, announced a technology transfer and licensing agreement with BMI Korea for its innovative injectable therapeutic, BxC-I17e. BxC-I17e, an exosome-based therapy currently undergoing clinical trials for the treatment of atopic dermatitis in the United States, is at the forefront of Brexogen’s pipeline. Under the terms of the agreement, BMI Korea gains rights to develop additional potential indications, excluding immune-related diseases, for domestic and major export markets.
Financially, Brexogen will receive a non-refundable upfront payment of 30 billion KRW (approximately $23 million) and will be eligible for milestone payments tied to clinical, regulatory, and commercial achievements, alongside double-digit percentage royalties on net annual sales. Additionally, if BMI Korea enters sublicensing agreements with third parties, Brexogen will share in the resultant licensing revenues. BMI Korea, a company generating over 100 billion KRW in annual revenue, brings robust pharmaceutical manufacturing capabilities, ensuring high-quality and scalable production for BxC-I17e.
BxC-I17e is a key asset in Brexogen's pipeline, leveraging the company’s proprietary exosome technology, which allows precise control over the therapeutic cargo carried by exosomes to enable targeted disease treatment. Currently undergoing Phase I clinical trials for atopic dermatitis, BxC-I17e demonstrates broad therapeutic potential. BMI Korea plans to explore its application in indications outside the immunology field, offering promising new therapeutic possibilities.
At present, no exosome-based therapies have received commercial approval globally, making this collaboration a significant step forward in advancing innovation in exosome therapeutic development and setting new benchmarks within the industry. Brexogen is not only exploring dermatological treatments with BxC-I17e but has already successfully commercialized products like BREXTEM-S for skin regeneration and BREXTEM-H for hair restoration. Looking ahead, Brexogen is preparing for its anticipated 2026 IPO on KOSDAQ, which is expected to accelerate the clinical and commercial adoption of exosome-based therapies.
9. Biogen and Stoke Therapeutics Collaborate on Developing a Treatment for Dravet Syndrome
On February 18, 2025, Biogen and Stoke Therapeutics announced a collaboration to develop and commercialize zorevunersen, a potentially first-in-class therapeutic for the treatment of Dravet syndrome. Dravet syndrome is a rare genetic epilepsy disorder characterized by treatment-resistant seizures and neurodevelopmental delays. Under the agreement, Stoke will continue to lead global development and retain exclusive development and commercialization rights in the U.S., Canada, and Mexico, while Biogen will have exclusive commercialization rights in other territories worldwide. The partnership leverages Biogen’s extensive expertise in neurological disorders and its global commercialization capabilities, aiming to bring new therapeutic hope to patients with Dravet syndrome.
As part of this collaboration, Stoke will receive an upfront payment of $165 million, and both companies will share external clinical development costs, with Biogen contributing 30% and Stoke covering 70%. Additionally, Stoke is eligible for up to $385 million in development and commercial milestone payments. If zorevunersen achieves commercial success globally, Stoke will receive tiered royalties on net sales, ranging from low double digits to high teens. This financial structure not only provides Stoke with the necessary funding support but also lays the groundwork for subsequent research and development activities.
Zorevunersen is an investigational antisense oligonucleotide (ASO) targeting the SCN1A gene, which is the root cause of most Dravet syndrome cases. The drug has been granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration (FDA) based on preliminary clinical evidence suggesting it can provide significant improvements unmet by existing therapies. In Phase I/IIa and open-label extension (OLE) studies, patients treated with zorevunersen experienced a sustained reduction in the frequency of convulsive tonic-clonic seizures, as well as improvements across multiple cognitive and behavioral metrics. These findings support the potential for zorevunersen to progress to pivotal global Phase III trials, potentially becoming the first treatment to alter the course of Dravet syndrome.
Currently, there are no approved disease-modifying therapies for Dravet syndrome, making the development of zorevunersen highly significant. The pivotal Phase III EMPEROR trial is expected to commence in the second quarter of 2025, with primary data readouts anticipated in the second half of 2027 to support global regulatory submissions. This progress is crucial for addressing the significant unmet medical needs of patients with Dravet syndrome and may herald a new era in the treatment of severe genetic disorders.
10. Foundation Medicine Partners with Sumitomo Pharma America to Advance Innovative Treatments for Acute Leukemia
On February 18, 2025, Foundation Medicine, a genomics company dedicated to transforming cancer care, announced a collaboration with Sumitomo Pharma America (SMPA) to develop FoundationOne® Heme as a companion diagnostic tool to identify acute leukemia patients with KMT2A rearrangements or NPM1 mutations. These patients may be eligible for treatment with SMPA’s investigational menin inhibitor, enzomenib (DSP-5336). The collaboration aims to leverage advanced comprehensive genomic analysis to develop novel targeted therapies for hematologic malignancies.
Acute leukemia is a condition requiring urgent intervention due to its rapid proliferation of blood cells, leading to the swift onset of symptoms. Approximately 30% of acute myeloid leukemia (AML) patients carry NPM1 mutations, while 5–10% of AML patients present with KMT2A (MLL) rearrangements. Menin inhibitors are gaining focus as a targeted therapeutic option for these specific genetic alterations. Menin plays a critical role in regulating hematopoiesis through its interaction with the KMT2A gene. The mechanism of action of menin inhibitors involves disrupting this protein-protein interaction, thereby inhibiting the proliferation of leukemia cells.
Enzomenib (DSP-5336), developed by Sumitomo Pharma, is a menin inhibitor designed to treat patients with KMT2A rearrangements or NPM1 mutations in acute leukemia. The drug works by interrupting the interaction between menin and KMT2A, aiming to inhibit the growth of leukemia cells. Preclinical studies have shown promising results for this therapeutic approach in patients with the aforementioned genetic variants. This partnership is expected to drive the development of enzomenib and accelerate its clinical application.
The FoundationOne® Heme platform combines DNA sequencing with RNA sequencing technologies, enabling the detection of all types of genomic alterations, including base substitutions, insertions and deletions, copy number variations, and gene fusions. This platform provides physicians with testing options for both blood and tissue samples, helping tailor personalized treatment plans based on a patient's specific genomic alterations.
Read the Rest of the Series
How to get the latest progress on drug deals?
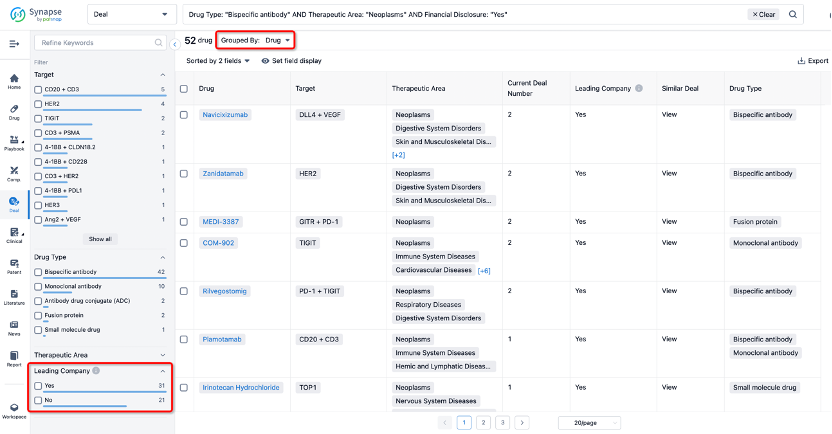
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
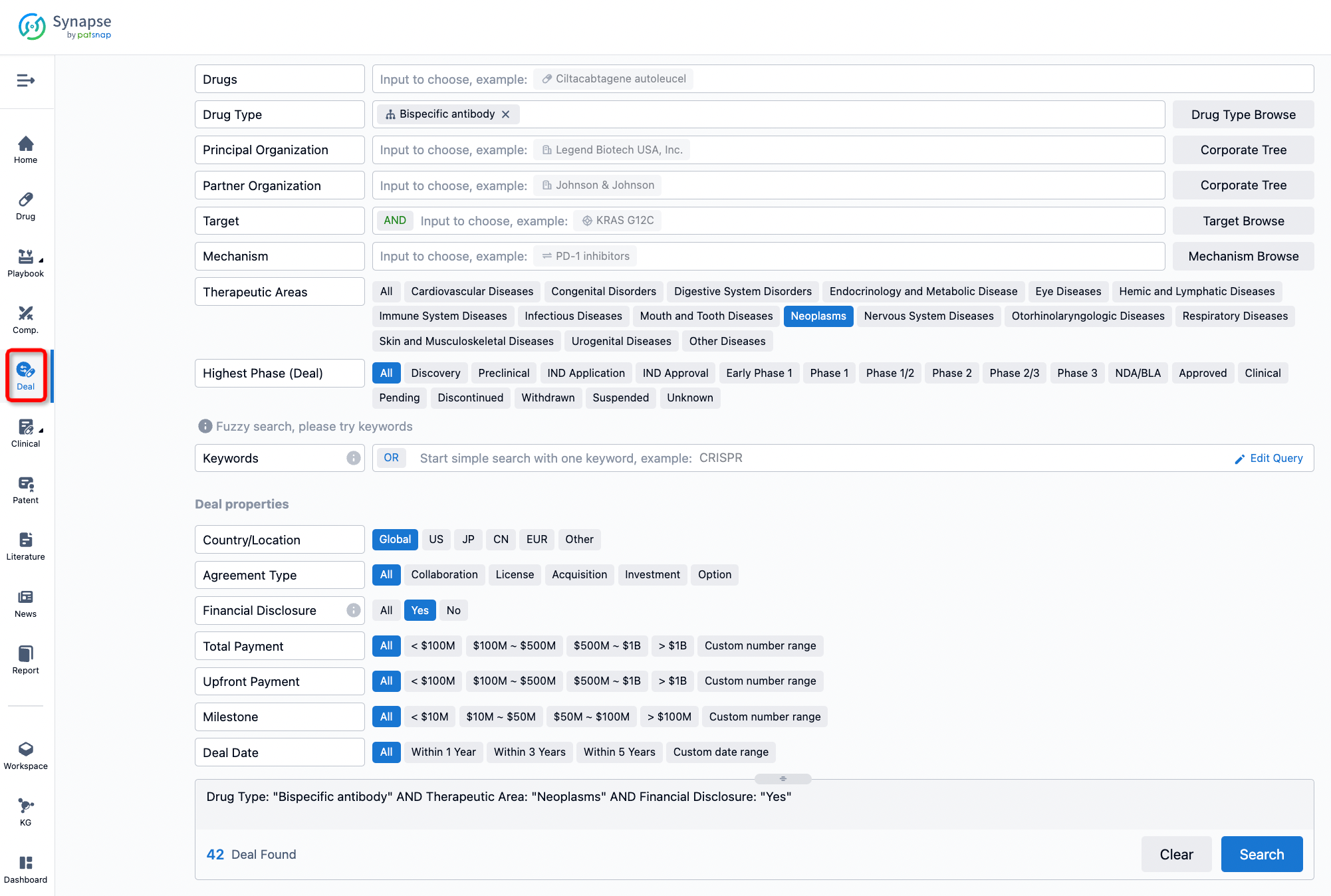
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
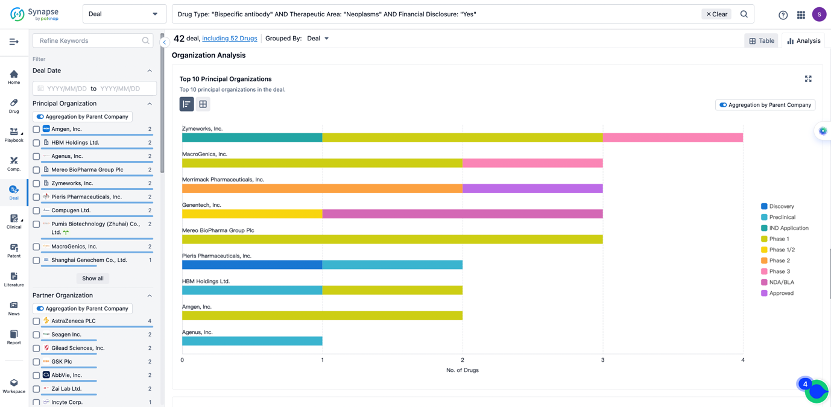
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
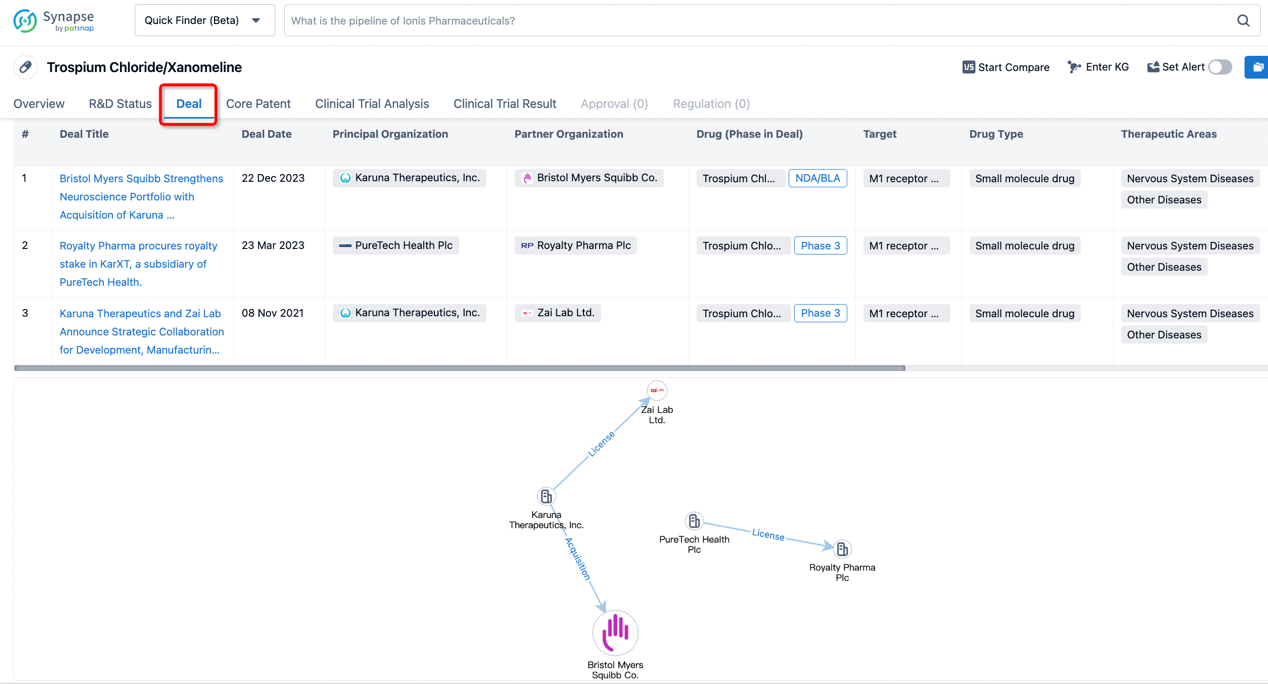
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
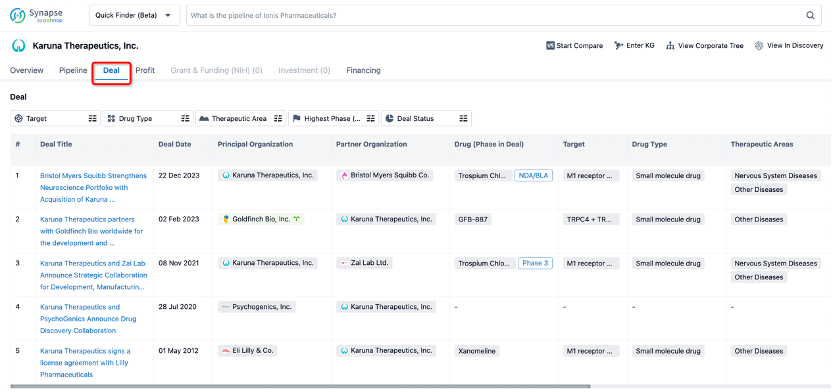
Click on the image below to explore new pharmaceutical funding transactions!