Unveiling the Secrets of JAK2 Inhibitors: Stay Updated with the Latest Advances?
JAK2, or Janus kinase 2, is an enzyme that plays a crucial role in the human body's signaling pathways. It is primarily involved in the activation of cytokine receptors, which are responsible for regulating various cellular processes. JAK2 is particularly important in hematopoiesis, the process of blood cell formation, as it is involved in the signaling of several key cytokines that control the production and differentiation of blood cells. Dysregulation or mutations in JAK2 can lead to various hematological disorders, including myeloproliferative neoplasms. Understanding the role of JAK2 is essential for developing targeted therapies to treat these disorders and potentially other diseases influenced by cytokine signaling.
Janus kinase 2 (JAK2) inhibitors are a type of medication that modulates the immune system by inhibiting the activity of the JAK2 enzyme, thereby interfering with the JAK-STAT signaling pathway in lymphocytes. They have revolutionized treatments for a range of disorders, such as myeloproliferative neoplasms, rheumatoid arthritis, inflammatory bowel disease, and multiple immune-driven dermatological diseases.
The development of JAK2 inhibitors began with the identification of potential inhibitors that work by blocking its tyrosine gated ATP binding site. The first JAK inhibitor to reach clinical trials was tofacitinib, a specific inhibitor of JAK3 that also inhibits JAK1 and JAK2 to a lesser extent.
The analysis of the current competitive landscape and future development of target JAK2 reveals a diverse range of companies, indications, drug types, and countries/locations involved in the development of JAK2-targeted drugs. Companies such as Incyte Corp., Eli Lilly & Co., Novartis AG, AbbVie, Inc., Pfizer Inc., GSK Plc, Bristol Myers Squibb Co., Swedish Orphan Biovitrum AB, Astellas Pharma, Inc., and Roche Holding AG are growing fastest under the current target, with drugs in various stages of development. The approved indications for JAK2-targeted drugs cover a wide range of diseases and conditions, indicating the potential therapeutic value of these drugs. Small molecule drugs and chemical drugs are progressing most rapidly, suggesting intense competition in the market. The countries/locations developing fastest include the United States, European Union, Japan, Norway, Liechtenstein, Iceland, China, Australia, Canada, United Kingdom, Switzerland, India, Kuwait, Spain, South Korea, Germany, Italy, France, Poland, and Israel. The progress in China highlights the country's growing presence in the pharmaceutical industry. Overall, the analysis indicates a competitive landscape and a promising future for the development of JAK2-targeted drugs.
How do they work?
JAK2 inhibitors are a type of medication that target the Janus kinase 2 (JAK2) enzyme. JAK2 is a protein involved in the signaling pathway of various cytokines and growth factors, playing a crucial role in the regulation of immune responses and blood cell production. These inhibitors work by blocking the activity of JAK2, thereby reducing the production of certain inflammatory mediators and suppressing abnormal cell growth.
From a biomedical perspective, JAK2 inhibitors are primarily used in the treatment of myeloproliferative neoplasms (MPNs), a group of blood disorders characterized by the overproduction of blood cells. One of the most well-known MPNs is polycythemia vera, where there is an excessive production of red blood cells. JAK2 inhibitors help to control the symptoms associated with these disorders, such as enlarged spleen, fatigue, and blood clotting complications.
Furthermore, JAK2 inhibitors have shown promise in the treatment of certain autoimmune diseases, such as rheumatoid arthritis and psoriasis, as well as in certain types of cancers, including certain forms of leukemia. By targeting the JAK2 enzyme, these inhibitors can help modulate the immune response and inhibit the abnormal cell growth associated with these conditions.
It is important to note that JAK2 inhibitors are prescription drugs and should only be used under the guidance of a healthcare professional. They may have potential side effects, such as an increased risk of infections, anemia, and liver toxicity. Regular monitoring and follow-up with a healthcare provider are necessary while using JAK2 inhibitors to ensure their safe and effective use.
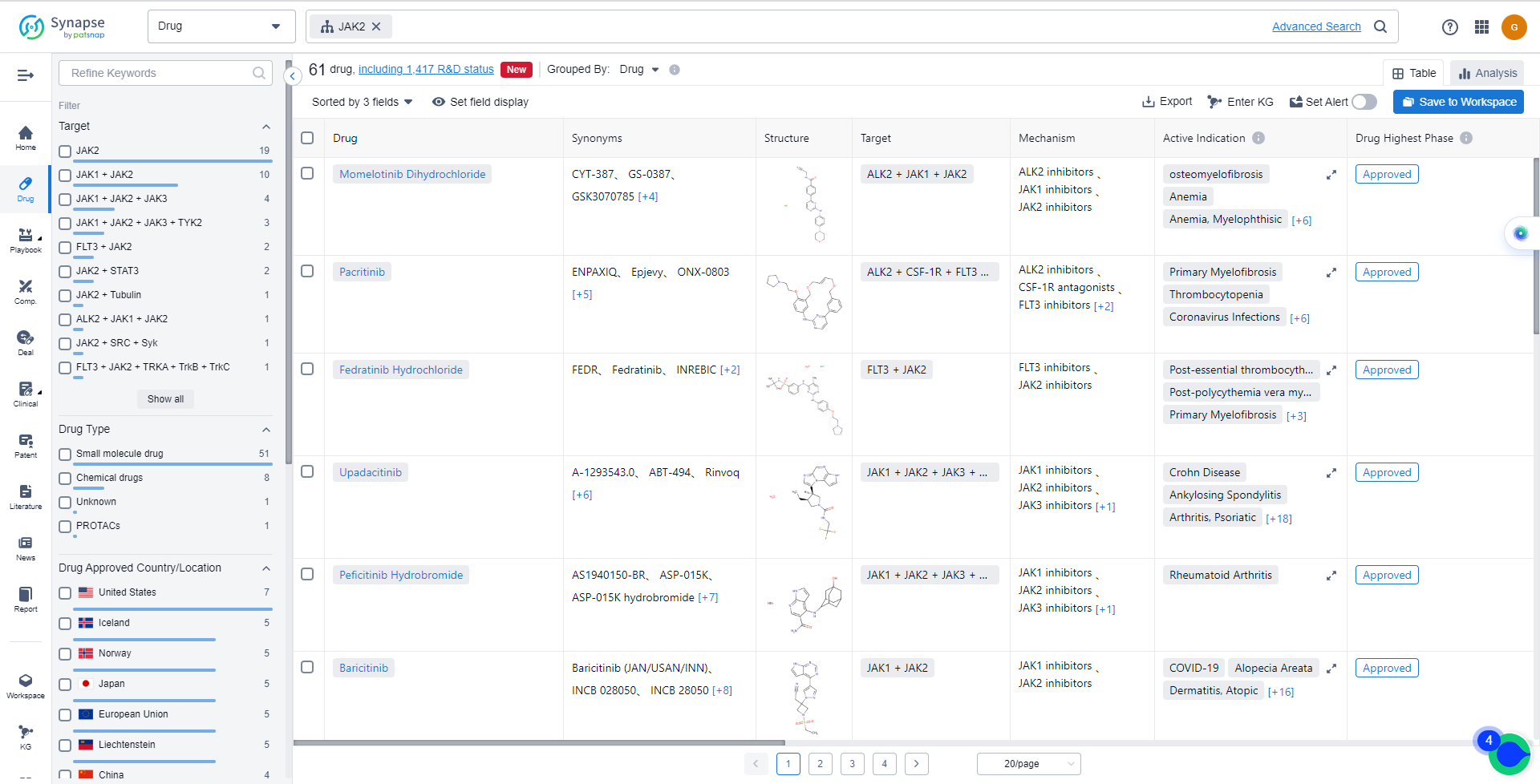
List of JAK2 Inhibitors
The currently marketed JAK2 inhibitors include:
- Momelotinib Dihydrochloride
- Pacritinib
- Fedratinib Hydrochloride
- Upadacitinib
- Peficitinib Hydrobromide
- Baricitinib
- Tofacitinib Citrate
- Ruxolitinib Phosphate
- Deuterated Ruxolitinib
- Lestaurtinib
For more information, please click on the image below.
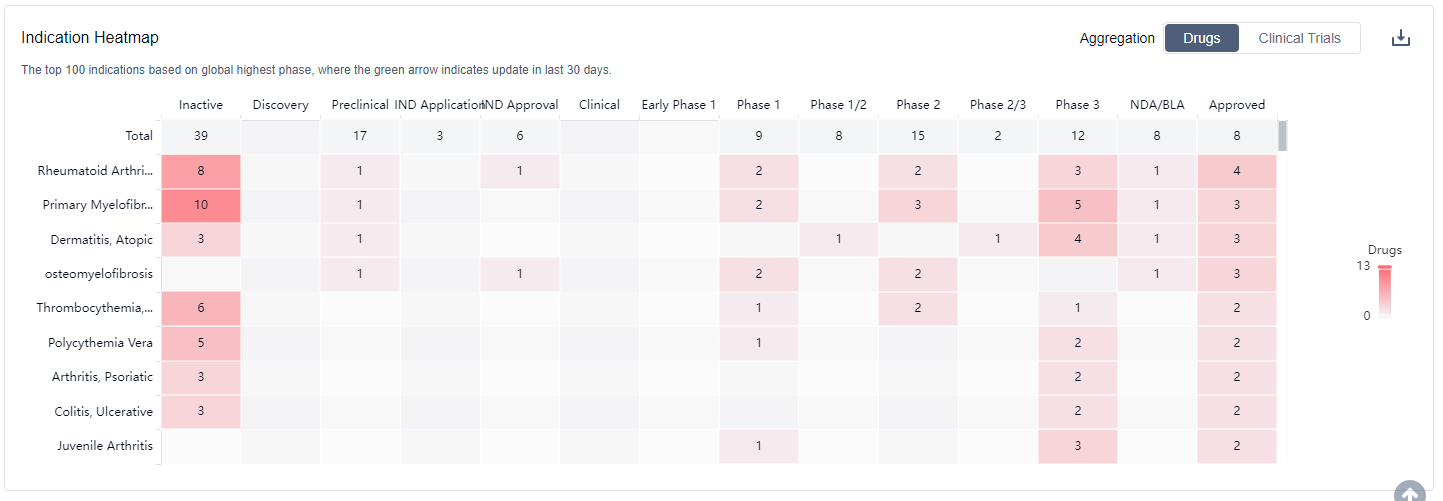
What are JAK2 inhibitors used for?
JAK2 inhibitors are primarily used in the treatment of myeloproliferative neoplasms (MPNs) For more information, please click on the image below to log in and search.
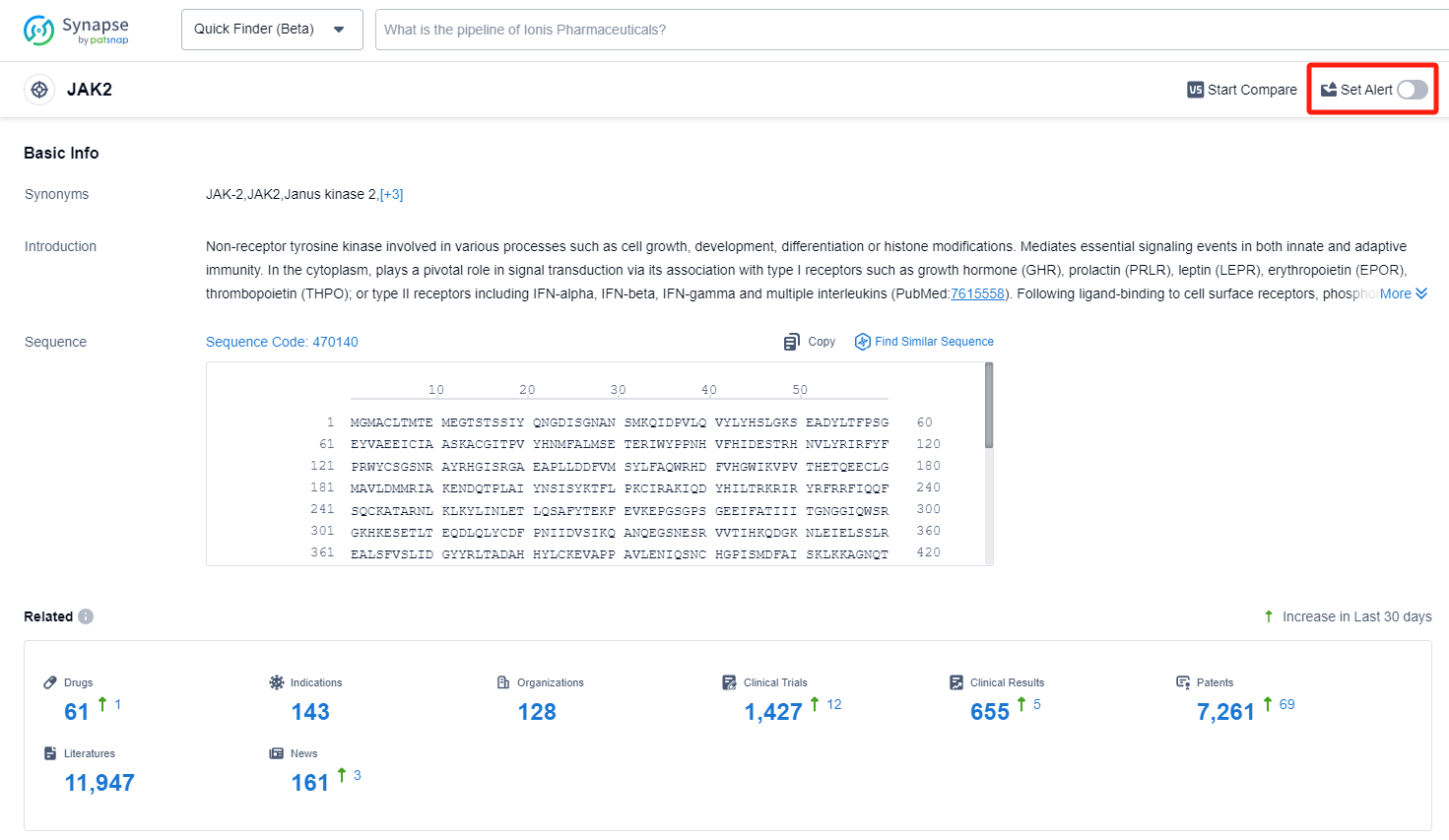
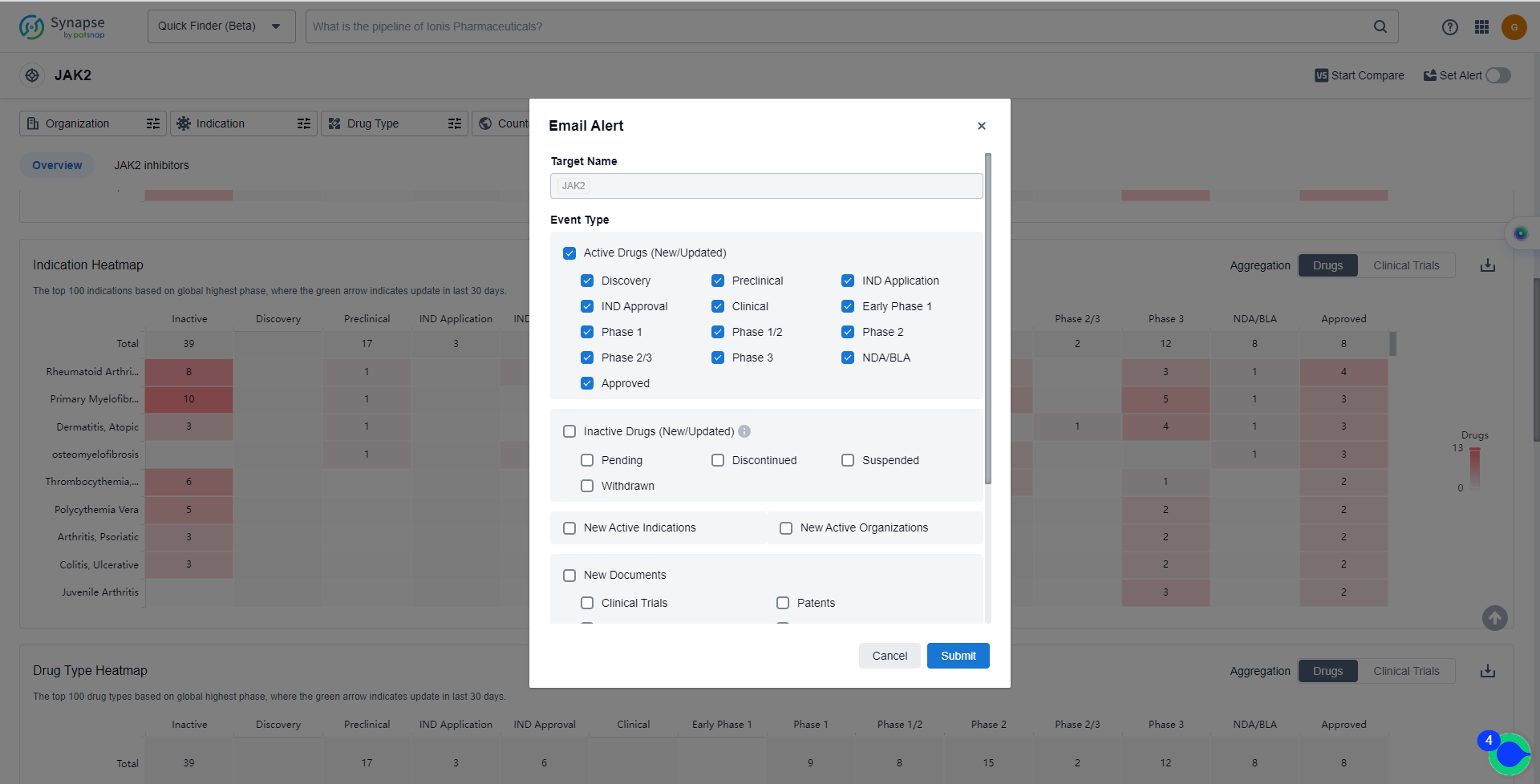
How to obtain the latest development progress of JAK2 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of JAK2 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!