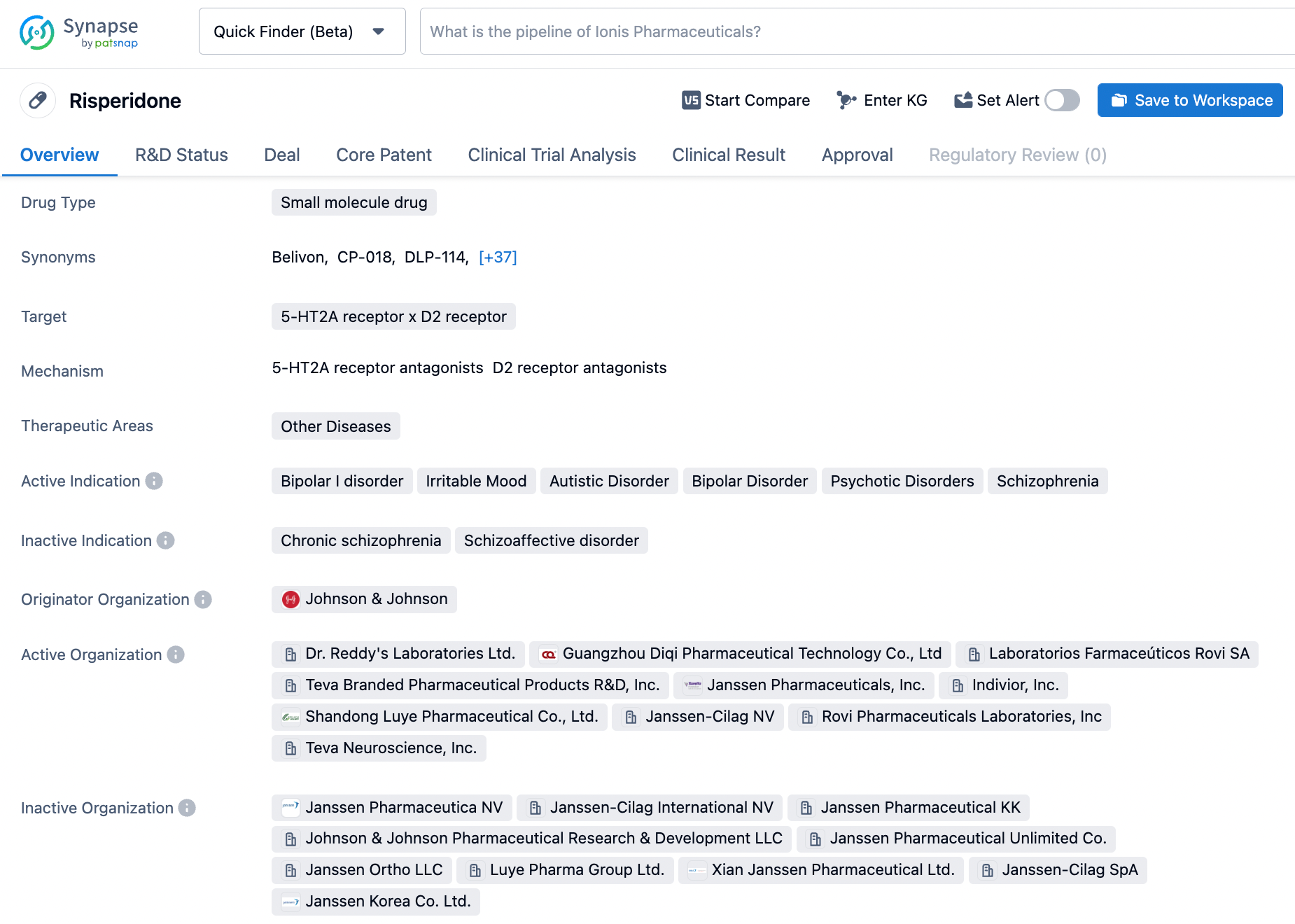
How to Effectively Search for Risperidone on Synapse
Risperidone, marketed under the trade name RISPERDAL®, is an atypical antipsychotic medication that was first approved by the US Food and Drug Administration (FDA) on December 29, 1993. Developed by Johnson & Johnson, risperidone is indicated for the treatment of schizophrenia, as well as acute manic or mixed episodes associated with bipolar I disorder, either as monotherapy or as an adjunctive therapy with lithium or valproate. It is also approved for the treatment of irritability associated with autism spectrum disorders. The drug's mechanism of action involves a combination of dopamine Type 2 (D2) and serotonin Type 2 (5HT2) receptor antagonism, which is believed to contribute to its therapeutic effects in treating schizophrenia. Risperidone is generally well-tolerated, but may cause side effects such as weight gain, drowsiness, and extrapyramidal symptoms. Click on the image below to begin the exploration journey of Risperidone through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!