How to find the chemical modification of Givosiran Sodium?
Givosiran Sodium, a groundbreaking therapeutic agent, is a small interfering RNA (siRNA) developed by Alnylam Pharmaceuticals, a leader in RNA interference (RNAi) technology. This innovative medication targets 5-aminolevulinate synthase 1 (ALAS1), an enzyme involved in the heme biosynthesis pathway, and is specifically indicated for the treatment of acute hepatic porphyrias (AHPs), a group of rare genetic disorders characterized by severe and potentially life-threatening symptoms such as abdominal pain, neuropathy, and hypertension. Givosiran Sodium, marketed under the brand name Givlaari, represents a significant advancement in the management of AHPs, offering a targeted and effective solution for patients with these debilitating conditions.
Summary of Research Progress of Givosiran Sodium
The research progress of Givosiran Sodium has been marked by significant achievements and milestones. Mechanistically, Givosiran Sodium operates through RNA interference (RNAi), a natural cellular process that can silence specific genes. By targeting the mRNA of ALAS1, Givosiran Sodium reduces the production of this enzyme, which in turn decreases the accumulation of toxic intermediates such as δ-aminolevulinic acid (ALA) and porphobilinogen (PBG). These intermediates are responsible for the painful and life-threatening symptoms associated with AHPs. The reduction in ALAS1 activity helps prevent the onset of acute attacks and manage chronic symptoms, significantly improving the quality of life for patients.
Givosiran Sodium has received regulatory approval in several key markets, including the United States, European Union, and Canada. The U.S. Food and Drug Administration (FDA) granted accelerated approval to Givosiran Sodium in November 2019, recognizing its breakthrough potential and the urgent need for effective treatments for AHPs. In the EU, Givosiran Sodium received a positive opinion from the European Medicines Agency (EMA) in January 2020, further expanding its availability to patients across Europe. These approvals were based on robust clinical trial data, particularly from the Phase III ENVISION trial, which demonstrated the efficacy and safety of Givosiran Sodium in reducing the frequency of AHP attacks and improving patient outcomes.
Globally, the competition in the AHP treatment market is limited, given the rarity and complexity of these conditions. However, Givosiran Sodium stands out as a first-in-class siRNA therapy, offering a novel and highly effective approach. Traditional treatments for AHPs, such as intravenous hemin, are often used to manage acute attacks but do not address the underlying cause of the disease. Givosiran Sodium, with its targeted mechanism of action, provides a more comprehensive and sustainable treatment option. The drug's unique profile has also attracted attention from healthcare providers and patient advocacy groups, highlighting its potential to transform the management of AHPs.
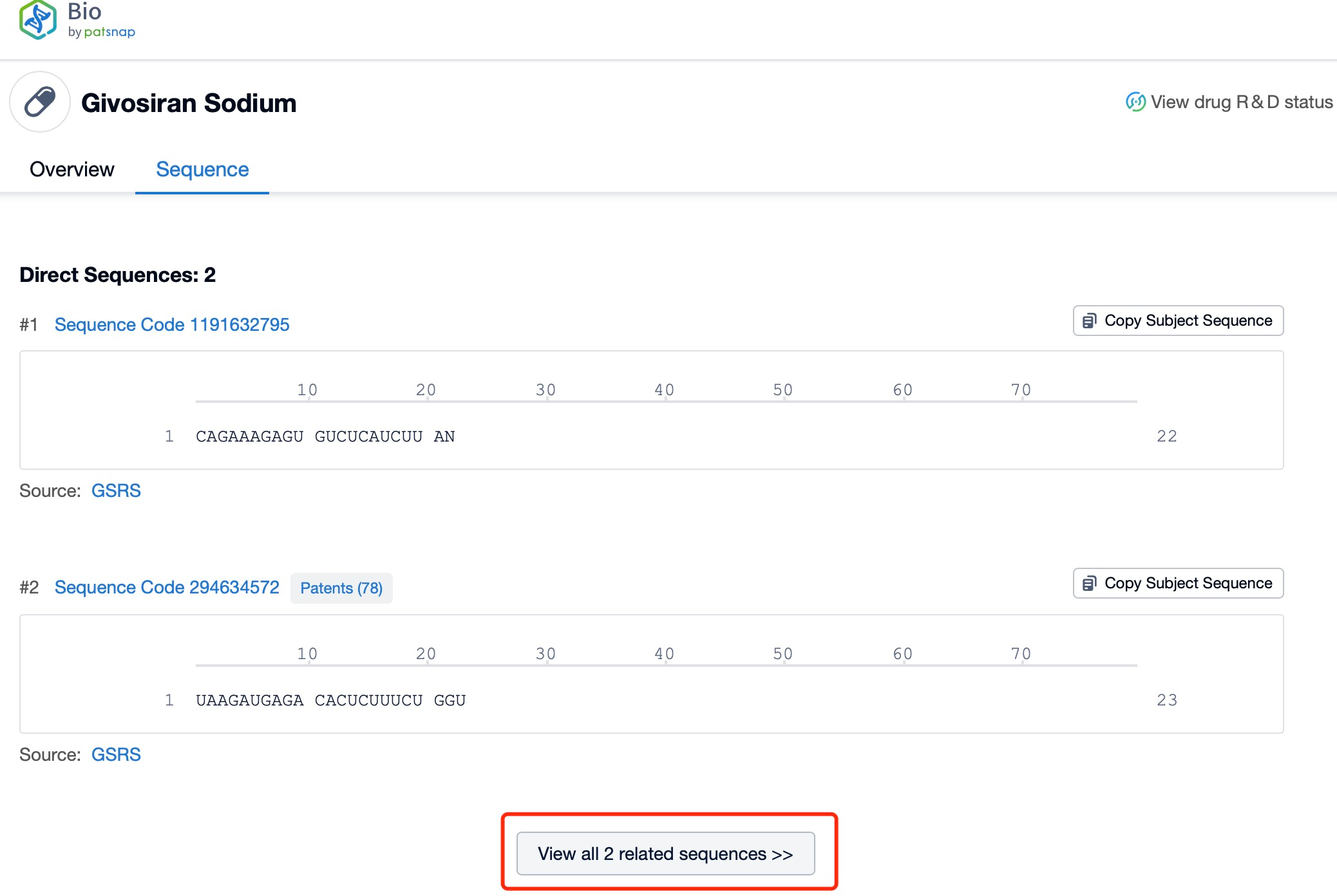
Sequence Information and Characteristics of Givosiran Sodium
The sequence information and characteristics of Givosiran Sodium are essential to understanding its mechanism of action and therapeutic potential. Givosiran Sodium is a chemically modified siRNA that targets the mRNA of ALAS1. The siRNA is designed to be a double-stranded RNA molecule, each strand approximately 21 nucleotides long. The sequence of the siRNA is meticulously engineered to ensure high specificity and affinity for the target mRNA, minimizing off-target effects and maximizing therapeutic efficacy. The antisense strand of the siRNA hybridizes with the ALAS1 mRNA, leading to its degradation and silencing of the ALAS1 gene.
One of the key features of Givosiran Sodium is its conjugation to N-acetylgalactosamine (GalNAc), a ligand that facilitates liver-specific delivery. The GalNAc-siRNA conjugate is recognized by the asialoglycoprotein receptor (ASGPR) on hepatocytes, enabling efficient uptake and intracellular processing. This targeted delivery system ensures that the siRNA reaches its intended site of action, the liver, where ALAS1 is predominantly expressed. The GalNAc conjugation also enhances the stability and bioavailability of the siRNA, allowing for a more potent and durable therapeutic effect.
Chemical Modification and Action of Givosiran Sodium
The chemical modifications of Givosiran Sodium further enhance its stability and efficacy. The siRNA component undergoes several modifications, including 2'-O-methoxyethyl (MOE) substitutions on the ribose sugar of certain nucleotides. These modifications increase the resistance of the siRNA to degradation by nucleases in the bloodstream, thereby extending its half-life and improving its stability. Additionally, the GalNAc conjugation improves the solubility and pharmacokinetic properties of the siRNA, allowing for a more stable and effective therapeutic molecule.
The action of Givosiran Sodium is mediated through the RNA interference (RNAi) pathway. Once inside the hepatocytes, the GalNAc-siRNA conjugate is processed by the RNA-induced silencing complex (RISC). The passenger strand of the siRNA is degraded, while the guide strand remains bound to the RISC. The guide strand then hybridizes with the complementary ALAS1 mRNA, leading to its cleavage and degradation. This process effectively silences the ALAS1 gene, reducing the production of ALAS1 protein and preventing the formation of toxic intermediates such as ALA and PBG. The reduction in ALAS1 levels is sustained over time, providing long-lasting therapeutic benefits and reducing the frequency and severity of AHP attacks.
Summary and Prospect
In summary, Givosiran Sodium (Givlaari) represents a significant breakthrough in the treatment of acute hepatic porphyrias (AHPs). Its targeted mechanism of action, involving the silencing of ALAS1 mRNA, offers a novel and effective approach to managing these rare and debilitating conditions. The drug's favorable safety profile, combined with its convenient dosing schedule of once-monthly subcutaneous injections, makes it an attractive option for patients with AHPs. With regulatory approvals in multiple regions and strong clinical trial data, Givosiran Sodium is poised to play a crucial role in the future of AHP treatment. Ongoing research and development efforts aim to further optimize the drug's therapeutic potential and explore its use in other related diseases, opening up new possibilities for patients suffering from genetic disorders and metabolic conditions.
How to find the chemical modification of all siRNAs?
In Patsnap Bio, you can find the sequence and latest research and development advances of all siRNAs.
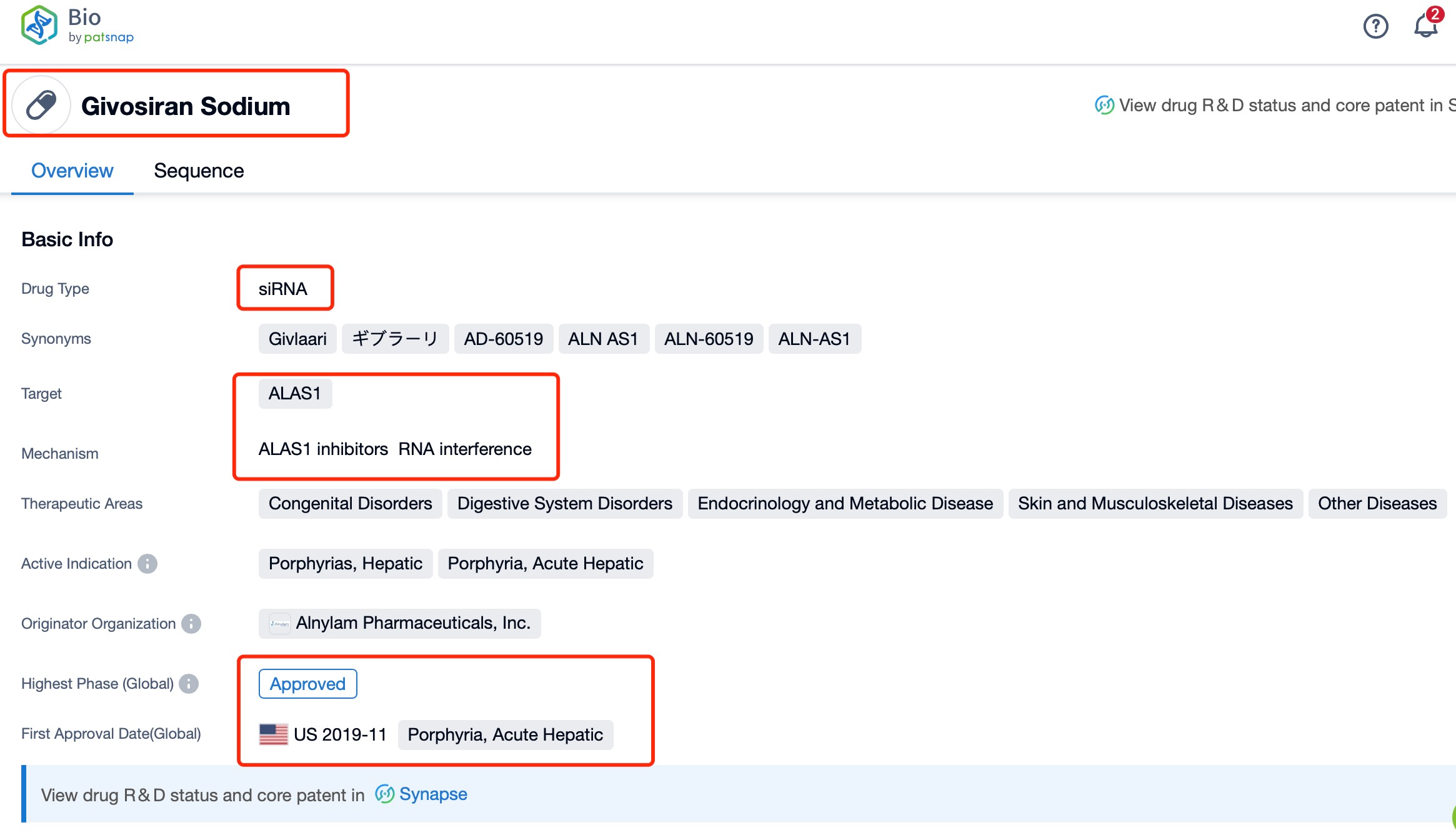
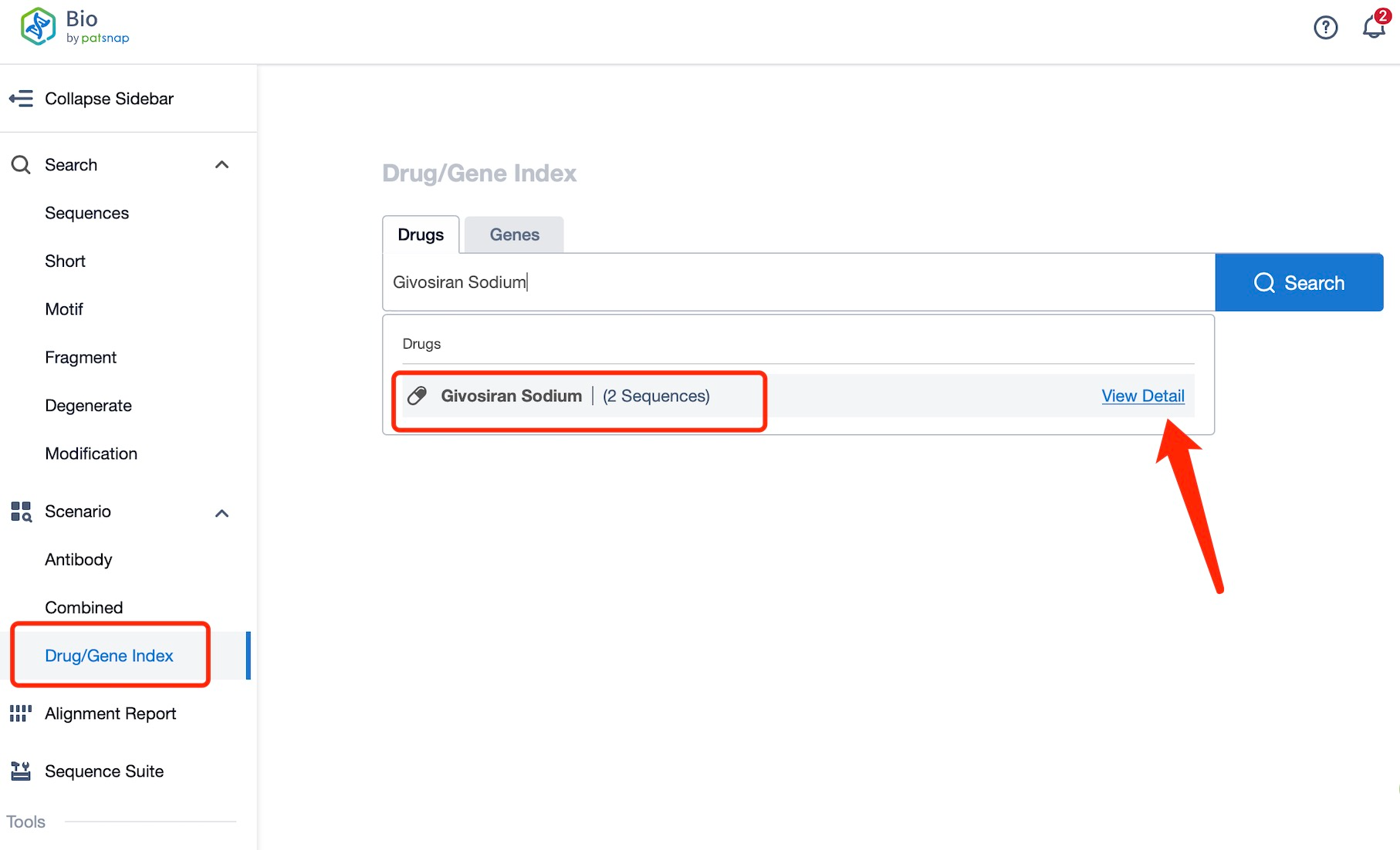
Taking Givosiran Sodium as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Givosiran Sodium ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Givosiran Sodium.
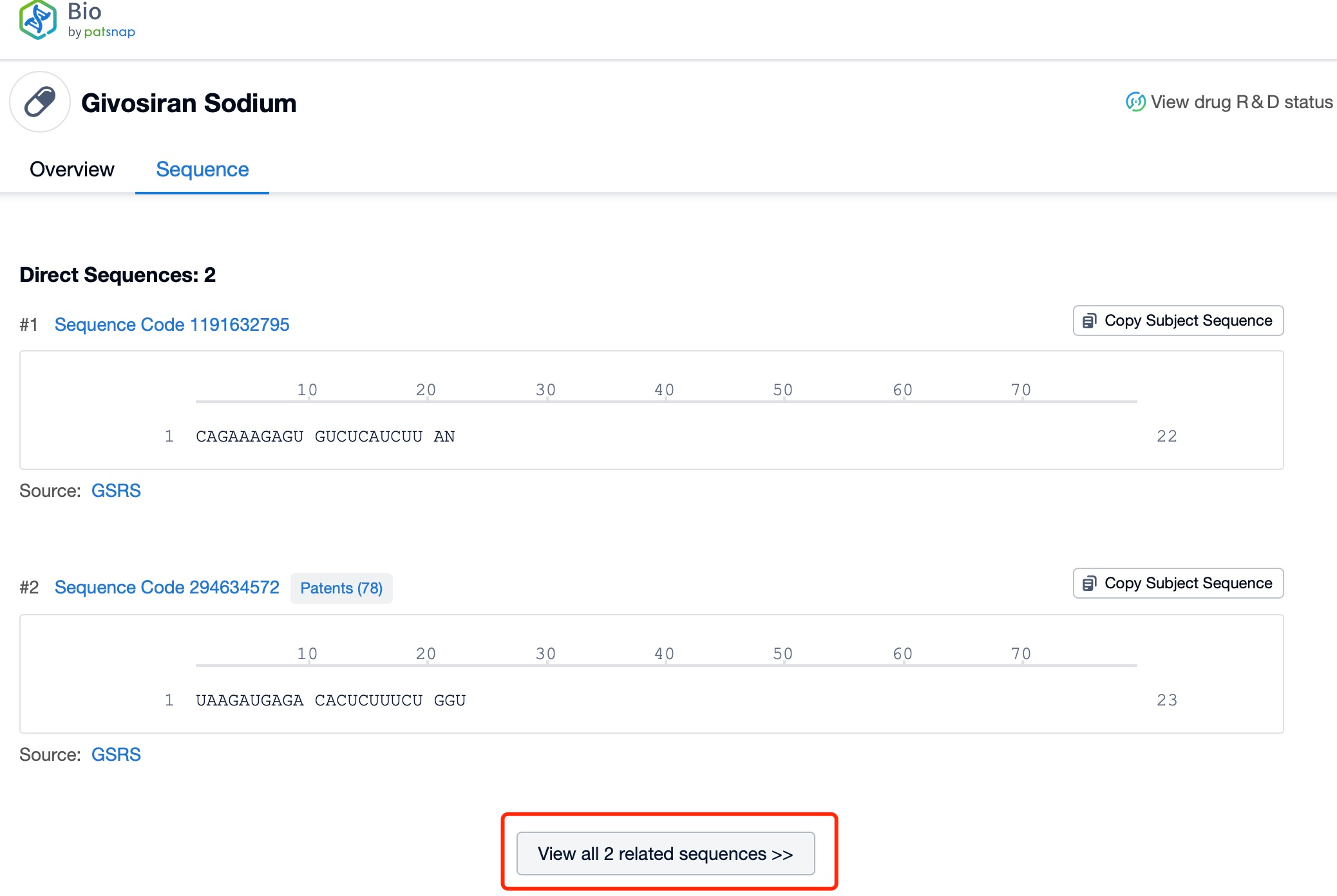
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
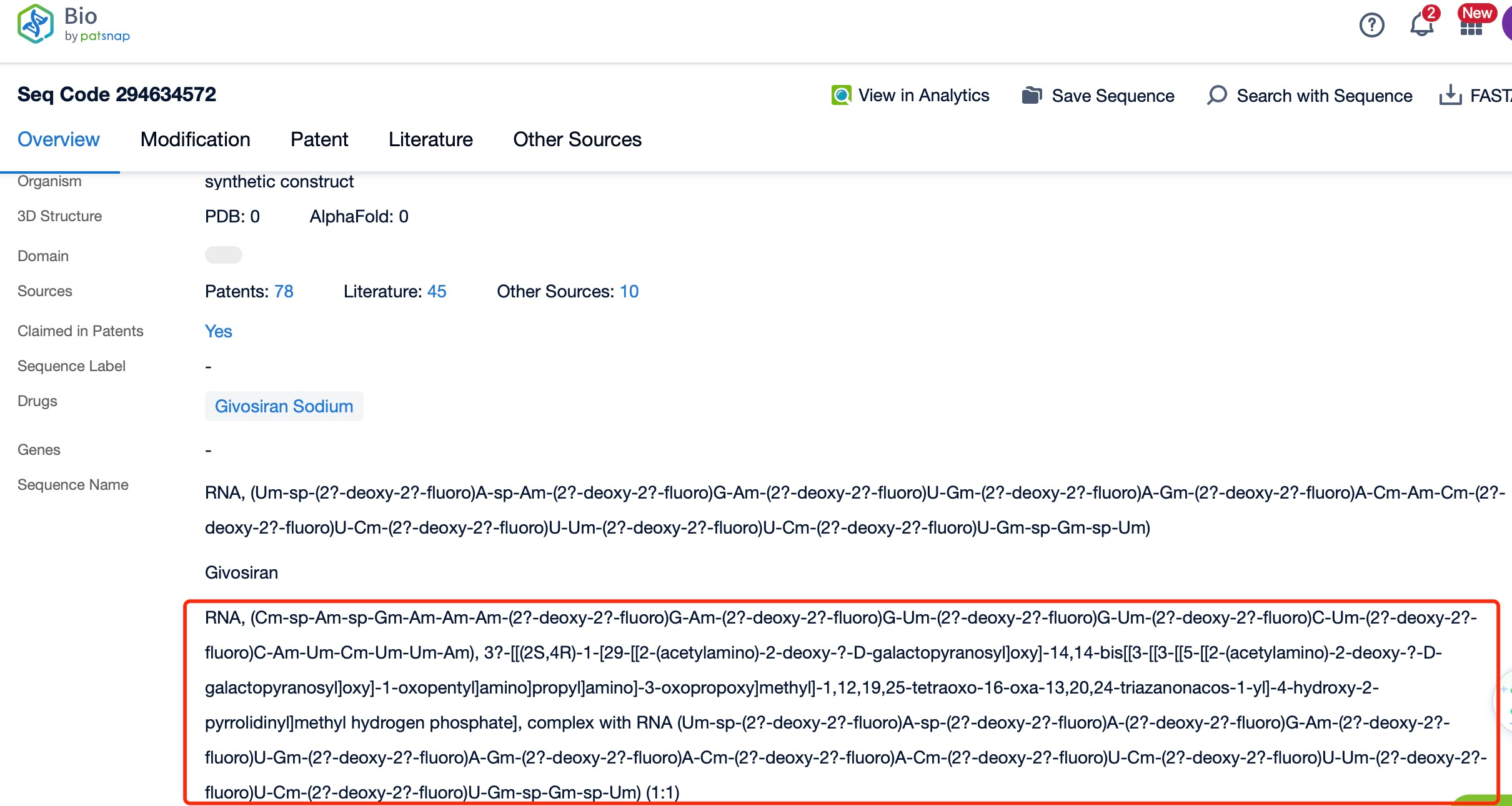
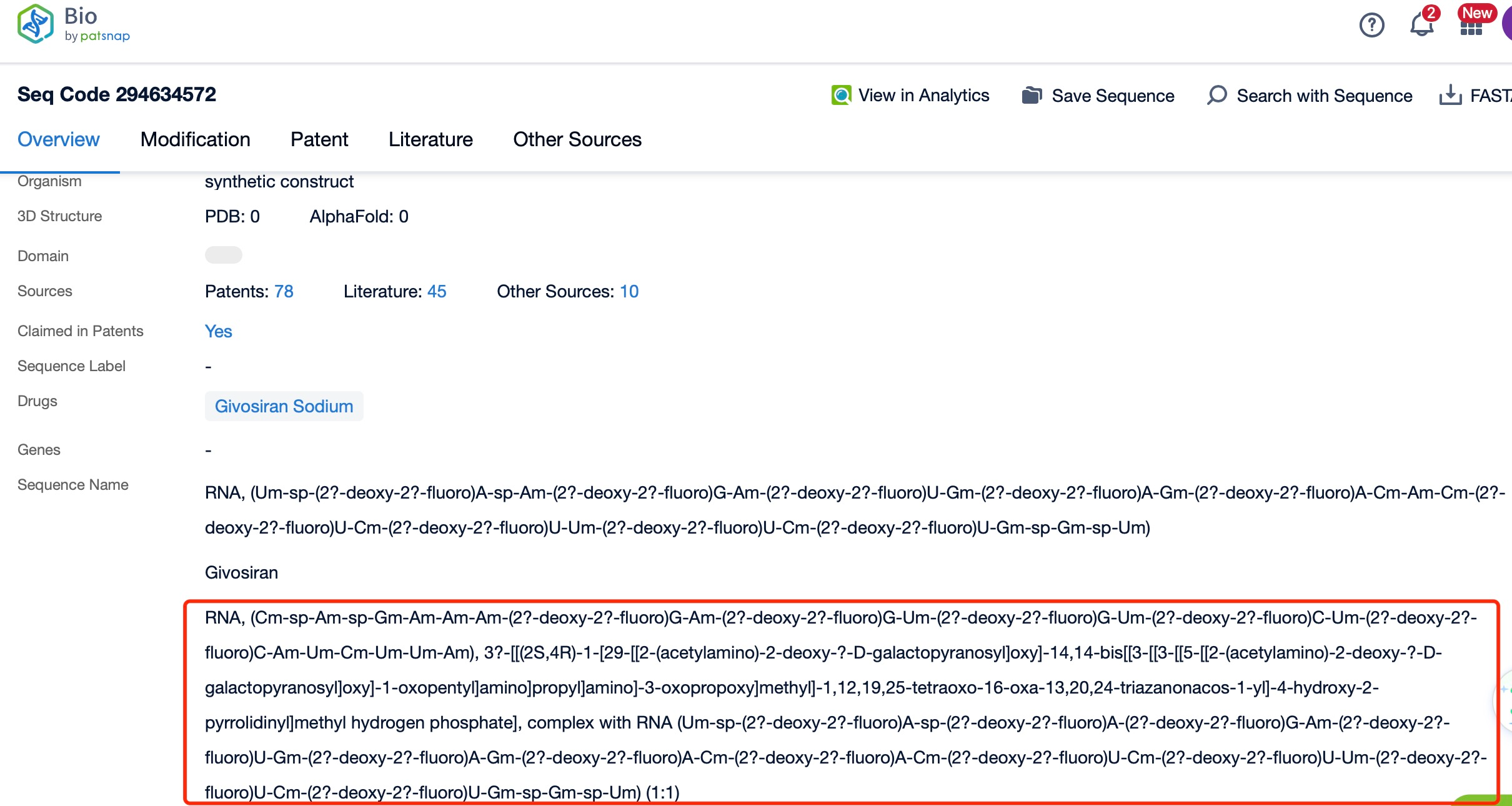
Clicking on the sequence name will provide you with all the basic information of that sequence. 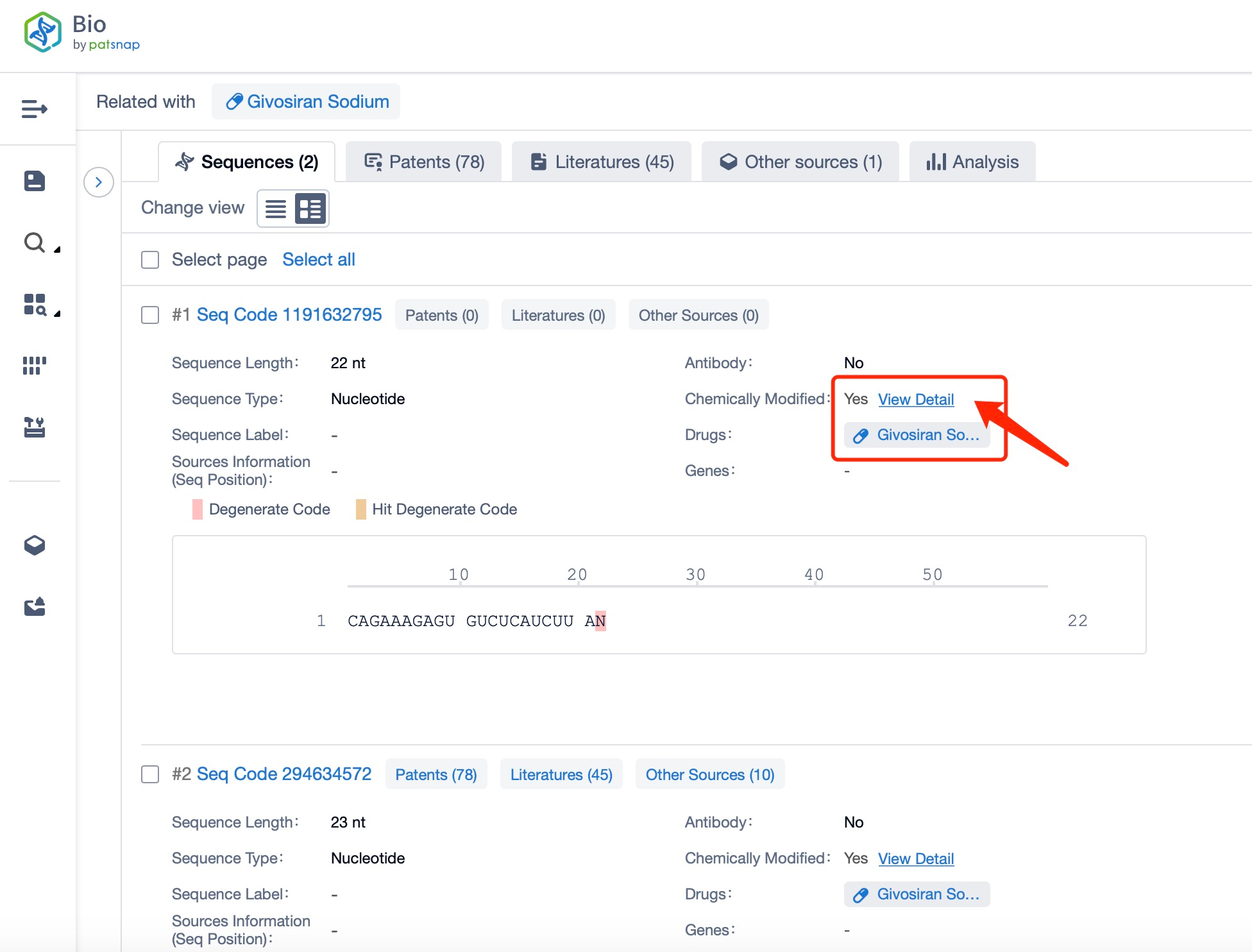
Additionally, a visual diagram of the sequence's chemical modifications is available for immediate access.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.