How to find the sequence of Casimersen?
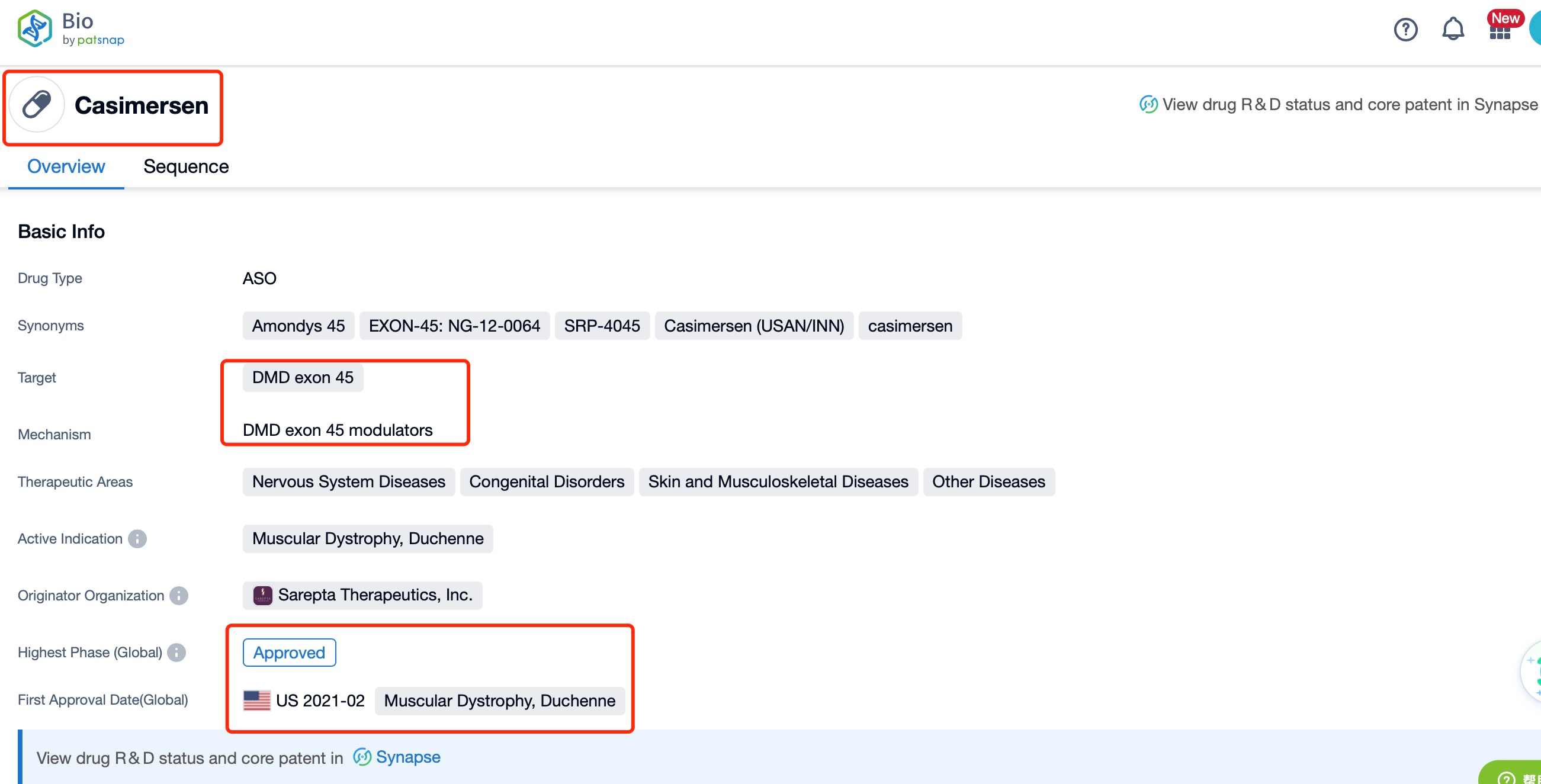
Casimersen, developed by Sarepta Therapeutics, is an antisense oligonucleotide (AON) designed to target and skip exon 45 of the dystrophin gene. Dystrophin is a protein that plays a crucial role in maintaining the integrity of muscle fibers, and mutations in the dystrophin gene are responsible for Duchenne muscular dystrophy (DMD), a severe and progressive muscle-wasting disease. Casimersen is indicated for the treatment of DMD in patients who have a confirmed mutation of the dystrophin gene that is amenable to exon 45 skipping. By inducing the skipping of exon 45 during mRNA processing, casimersen aims to restore the reading frame of the dystrophin gene, allowing for the production of a truncated but functional dystrophin protein.
Summary of Research Progress of Casimersen
Casimersen works through a mechanism known as exon skipping. It binds to a specific region of the pre-mRNA of the dystrophin gene, causing the splicing machinery to skip over exon 45 during the mRNA processing. This skipping restores the reading frame of the mRNA, enabling the production of a shortened but partially functional dystrophin protein. The restoration of dystrophin expression helps to stabilize muscle fibers, reduce muscle damage, and slow the progression of DMD. Casimersen is administered via subcutaneous injection, providing a convenient and less invasive route of administration compared to other therapeutic options.
Casimersen has been granted accelerated approval by the U.S. Food and Drug Administration (FDA) for the treatment of DMD in patients with a confirmed mutation of the dystrophin gene that is amenable to exon 45 skipping. The drug is currently under review by other regulatory agencies, including the European Medicines Agency (EMA), and is available in certain markets. Despite its approval, casimersen faces competition from other DMD therapies, including other exon-skipping AONs and gene therapies. For example, Sarepta Therapeutics also markets eteplirsen (exon 51 skipping) and golodirsen (exon 53 skipping), while other companies, such as Pfizer and Solid Biosciences, are developing gene therapies for DMD. However, casimersen offers a unique advantage for patients with mutations amenable to exon 45 skipping, a relatively rare subset of DMD patients.
Clinical research on casimersen has demonstrated its efficacy and safety in treating DMD. The Phase 3 clinical trial, known as ESSENCE, showed that casimersen could increase dystrophin protein levels in patients with DMD who have a confirmed mutation amenable to exon 45 skipping. The trial also indicated that casimersen could improve muscle function and delay the loss of ambulation, key endpoints in the management of DMD. The drug was generally well-tolerated, with common side effects including injection site reactions, headache, and upper respiratory tract infections. Ongoing and future trials aim to further evaluate the long-term safety and efficacy of casimersen, as well as explore its potential in combination with other therapies to achieve better outcomes.
Sequence Characteristics of Casimersen
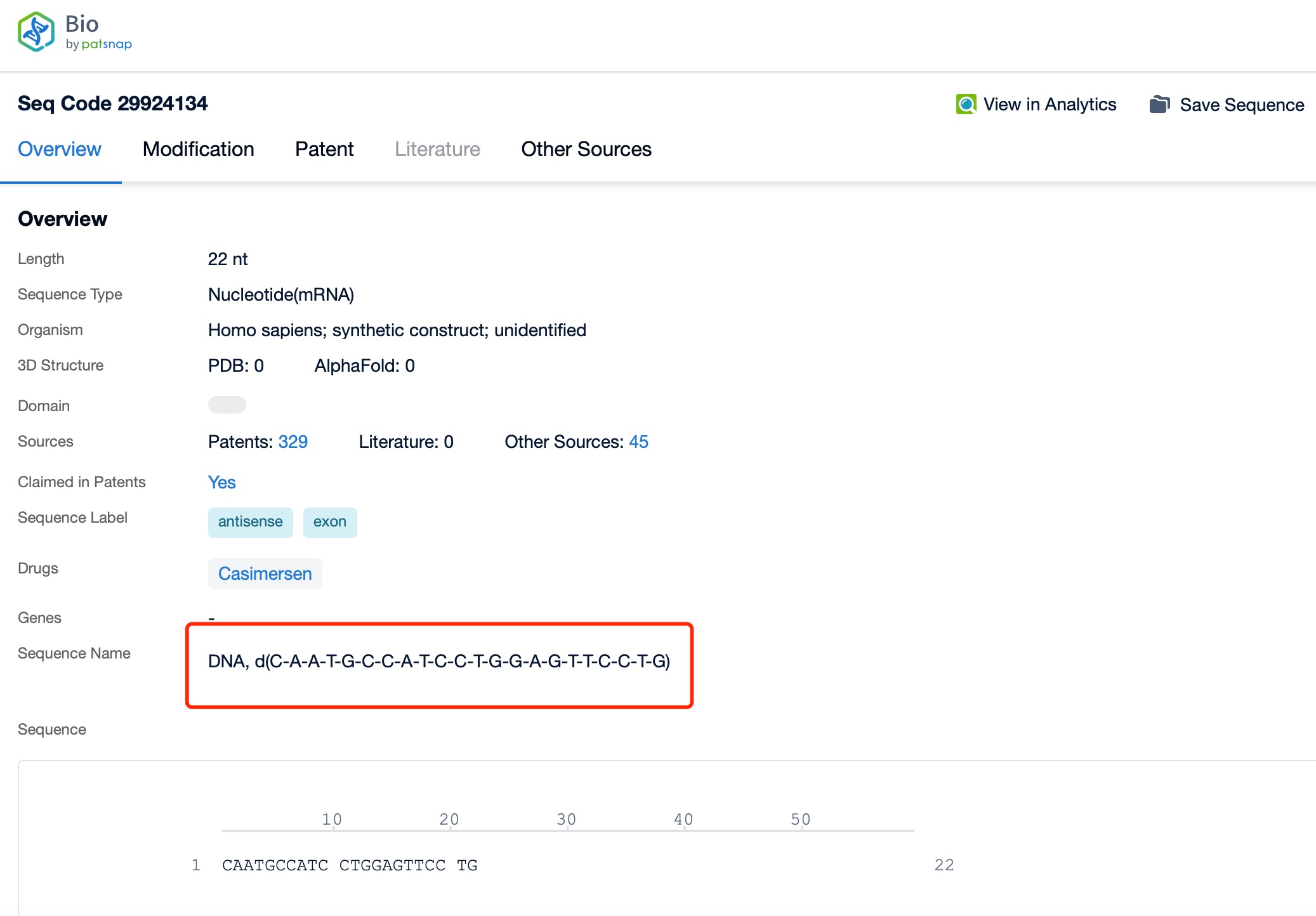
Casimersen is a 22-nucleotide phosphorothioate-modified oligonucleotide. The sequence of casimersen is specifically designed to bind to the pre-mRNA of the dystrophin gene at a region that corresponds to exon 45. This sequence is optimized to ensure high specificity and efficiency in binding to the target RNA, which is crucial for the drug's therapeutic efficacy and safety. The precise sequence of casimersen is a proprietary information, but it is known to be highly complementary to the target region of the dystrophin pre-mRNA, allowing for effective exon skipping.
Chemical Modification and Species of Casimersen
The chemical modifications in casimersen include phosphorothioate linkages. The phosphorothioate backbone replaces one of the non-bridging oxygen atoms in the phosphate group with a sulfur atom, enhancing the stability of the oligonucleotide and protecting it from nuclease degradation. This modification is crucial for the drug's ability to reach and bind to the target RNA in muscle cells. The phosphorothioate backbone also improves the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are important for its therapeutic effectiveness.
The phosphorothioate modifications in casimersen provide several key advantages. First, they significantly enhance the stability of the oligonucleotide, allowing it to remain active in the cellular environment for a longer period. This increased stability is crucial for the drug's effectiveness in binding to and skipping exon 45 of the dystrophin pre-mRNA. Second, the phosphorothioate modifications improve the pharmacokinetic profile of the drug, increasing its half-life and bioavailability. This means that the drug can be administered less frequently, which is beneficial for patient convenience and compliance. Third, the phosphorothioate modifications reduce the risk of off-target effects by enhancing the specificity of the oligonucleotide for its target RNA.
The role of the phosphorothioate modifications in casimersen is multifaceted. They protect the oligonucleotide from degradation by nucleases, ensuring that it can reach its target in muscle cells. They also enhance the binding affinity of the oligonucleotide to the dystrophin pre-mRNA, ensuring efficient and specific exon skipping. Additionally, the phosphorothioate modifications improve the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are essential for its therapeutic effectiveness. These modifications also reduce the immunogenicity of the oligonucleotide, minimizing the risk of adverse immune responses.
Summary and Prospect
In summary, casimersen represents a significant advancement in the treatment of Duchenne muscular dystrophy (DMD) for patients with mutations amenable to exon 45 skipping. Its mechanism of action, involving the skipping of exon 45 to restore the reading frame of the dystrophin gene, has shown promising results in increasing dystrophin protein levels and improving muscle function. Despite facing competition from other DMD therapies, casimersen offers a unique advantage for a specific subset of DMD patients. Future research may focus on improving delivery methods and exploring combination therapies to enhance its clinical utility. The sequence characteristics and chemical modifications of casimersen, including its phosphorothioate backbone, contribute to its stability, specificity, and efficacy, making it a valuable tool in the management of DMD. However, the need for regular subcutaneous injections and the management of side effects will continue to be important considerations in its clinical use. The ongoing and future clinical trials will provide further insights into the long-term safety and efficacy of casimersen, potentially paving the way for its broader use in the treatment of DMD.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
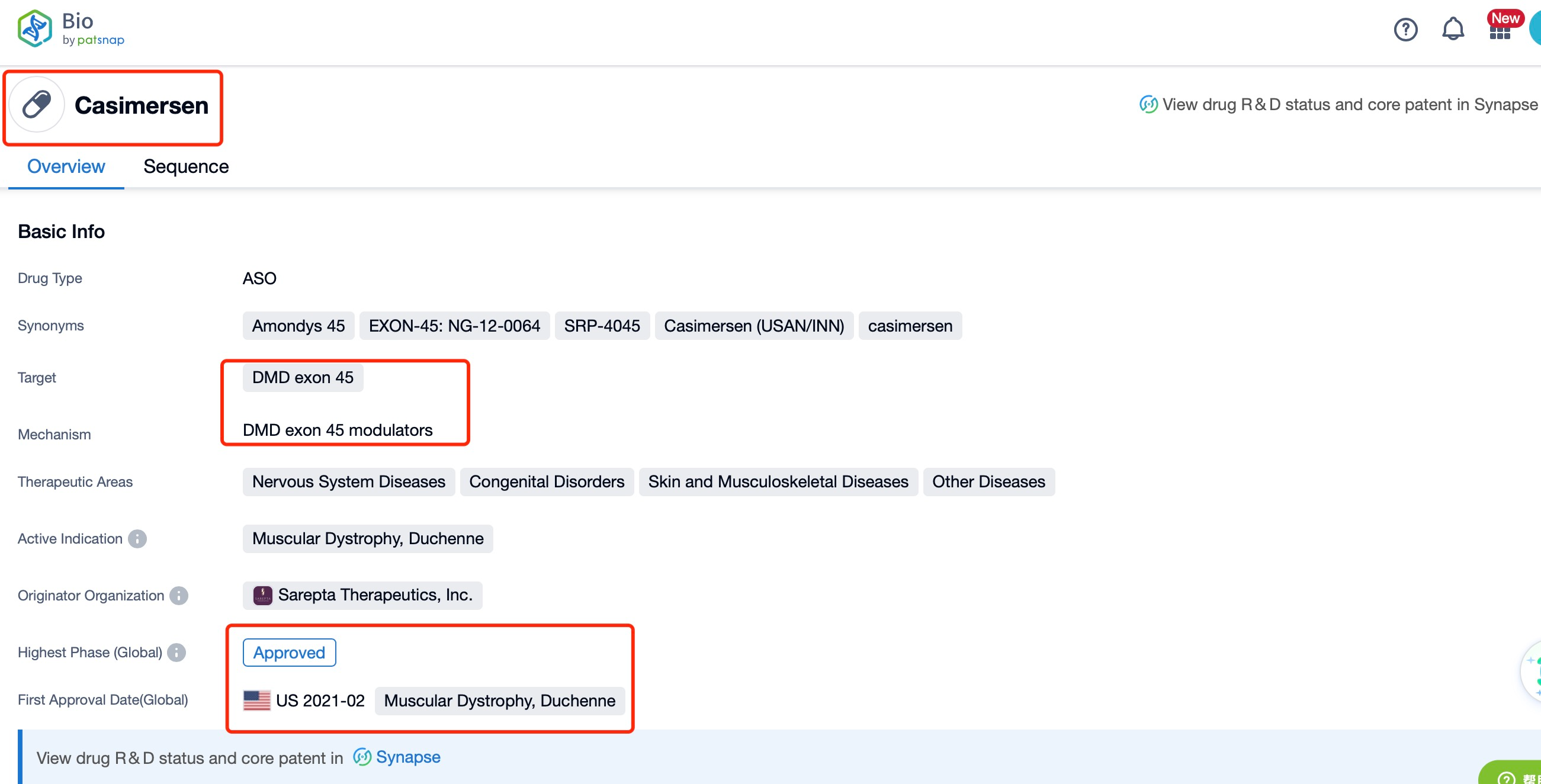
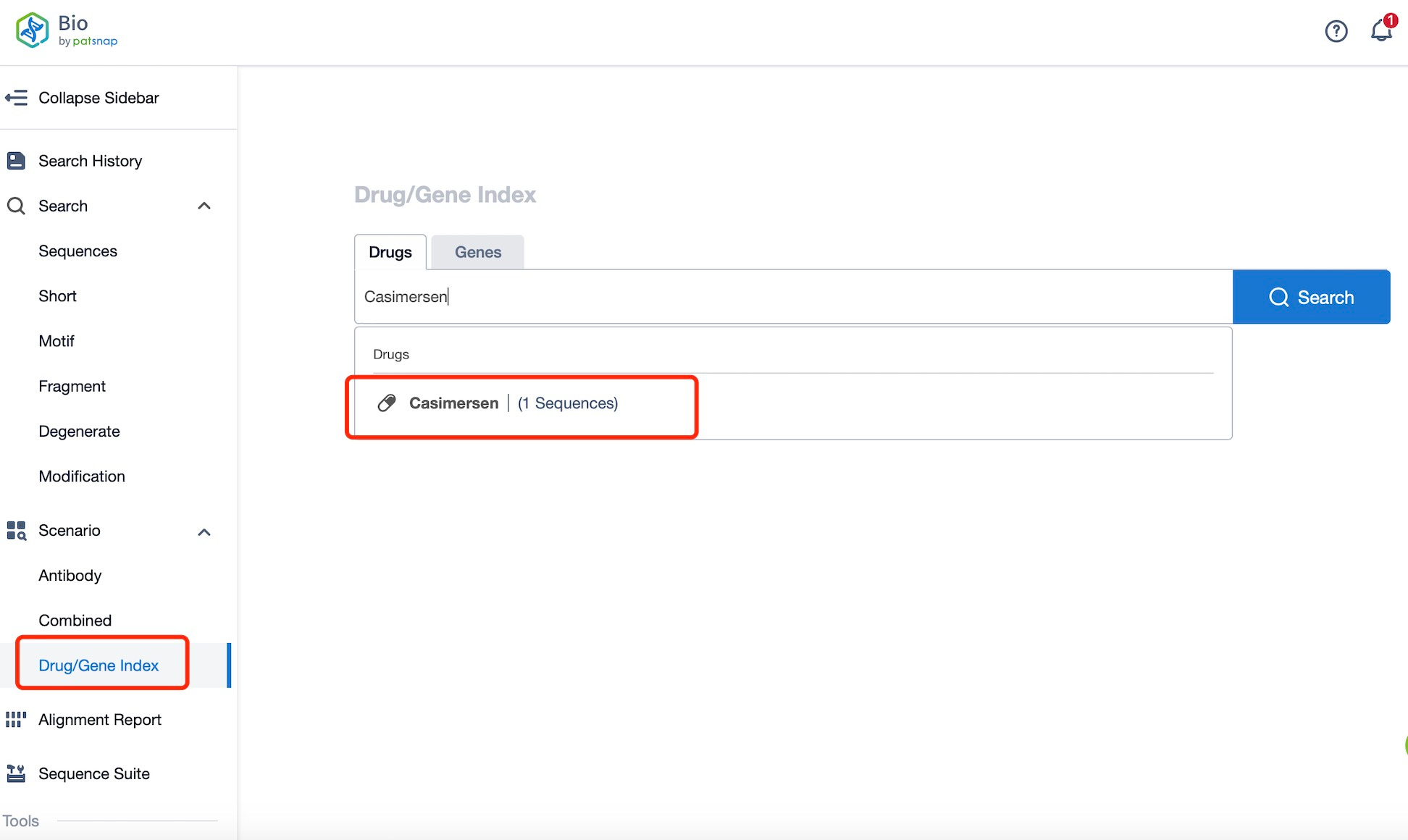
Taking casimersen as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' casimersen ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of casimersen.
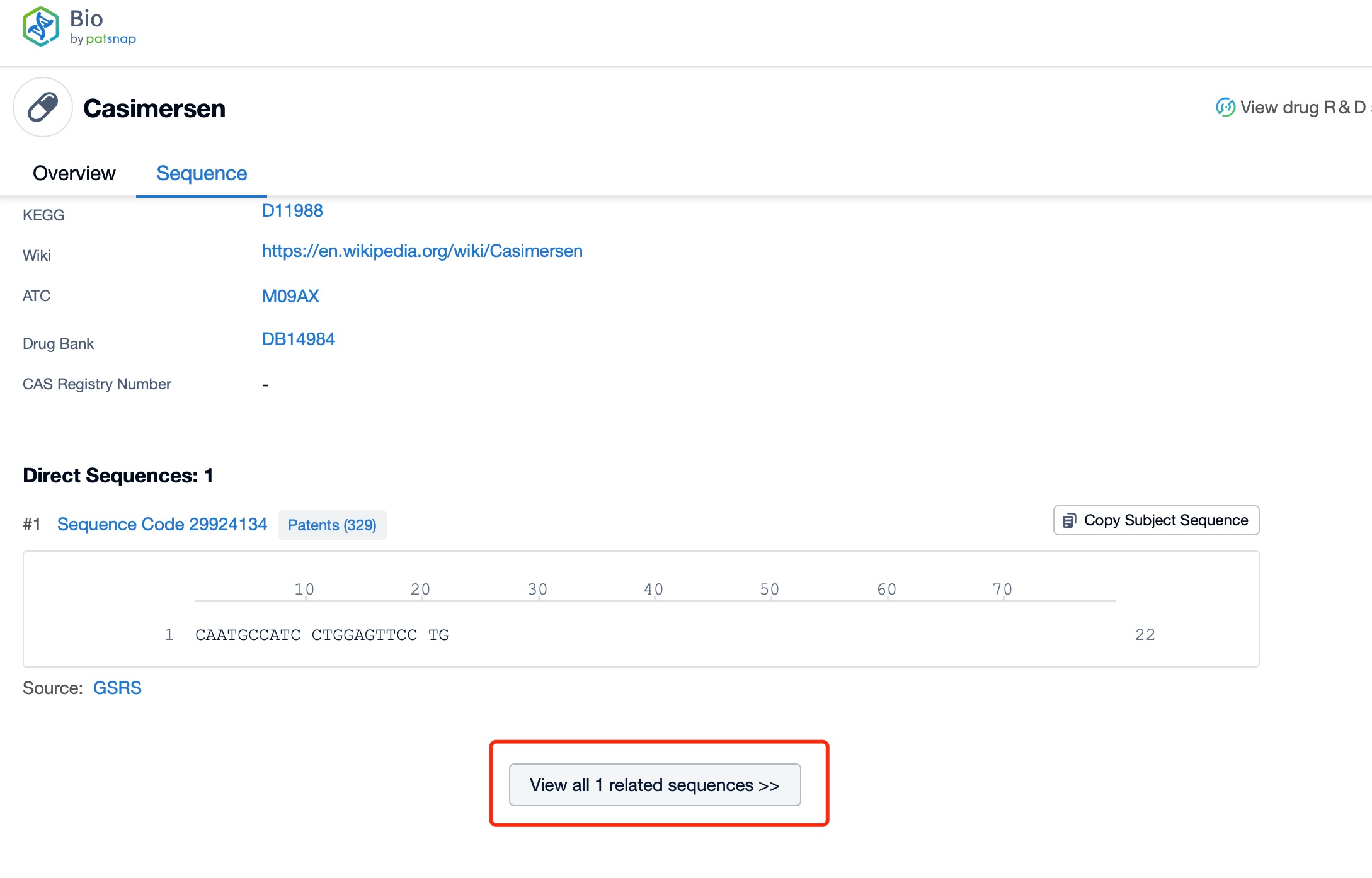
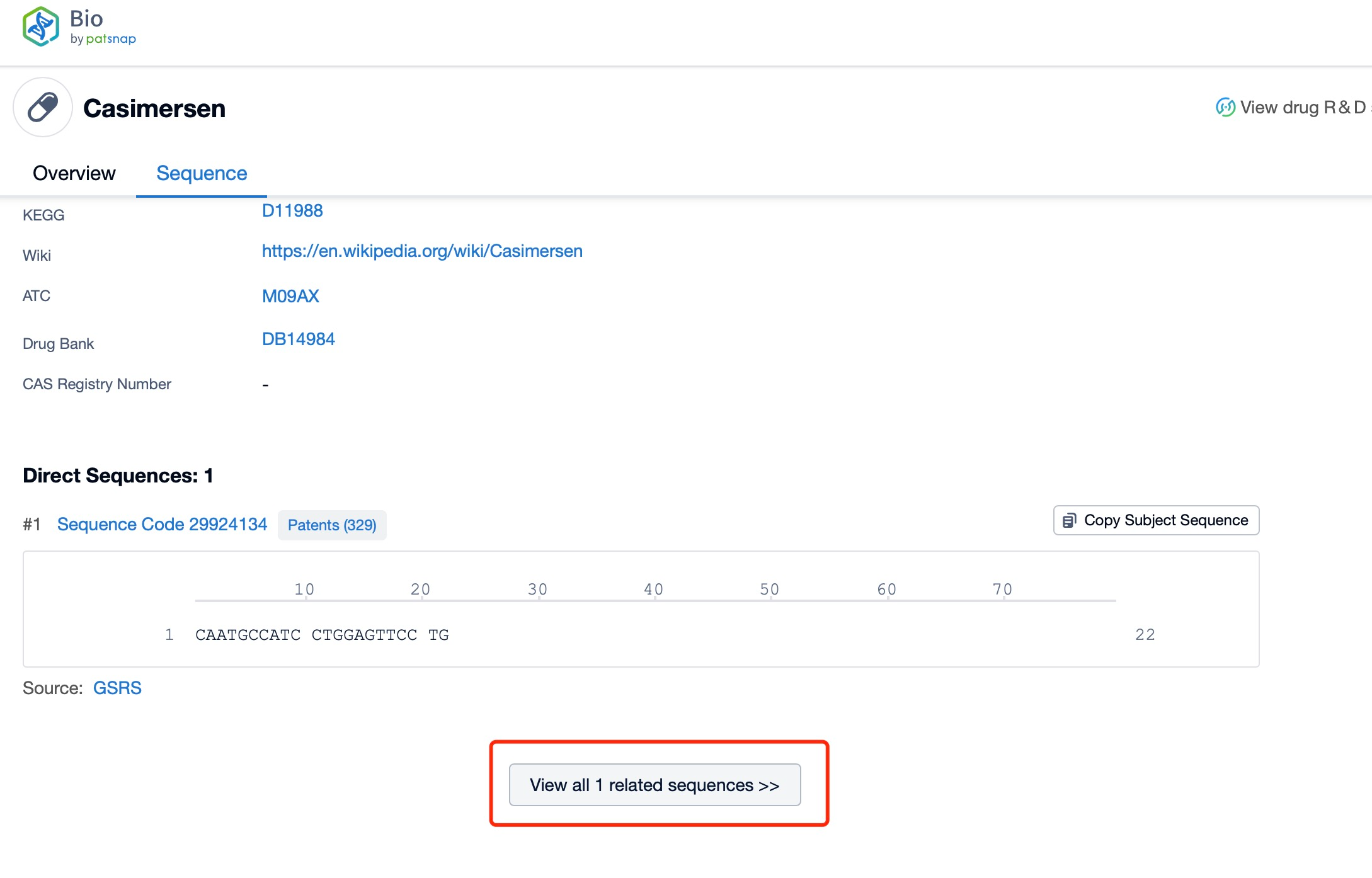
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
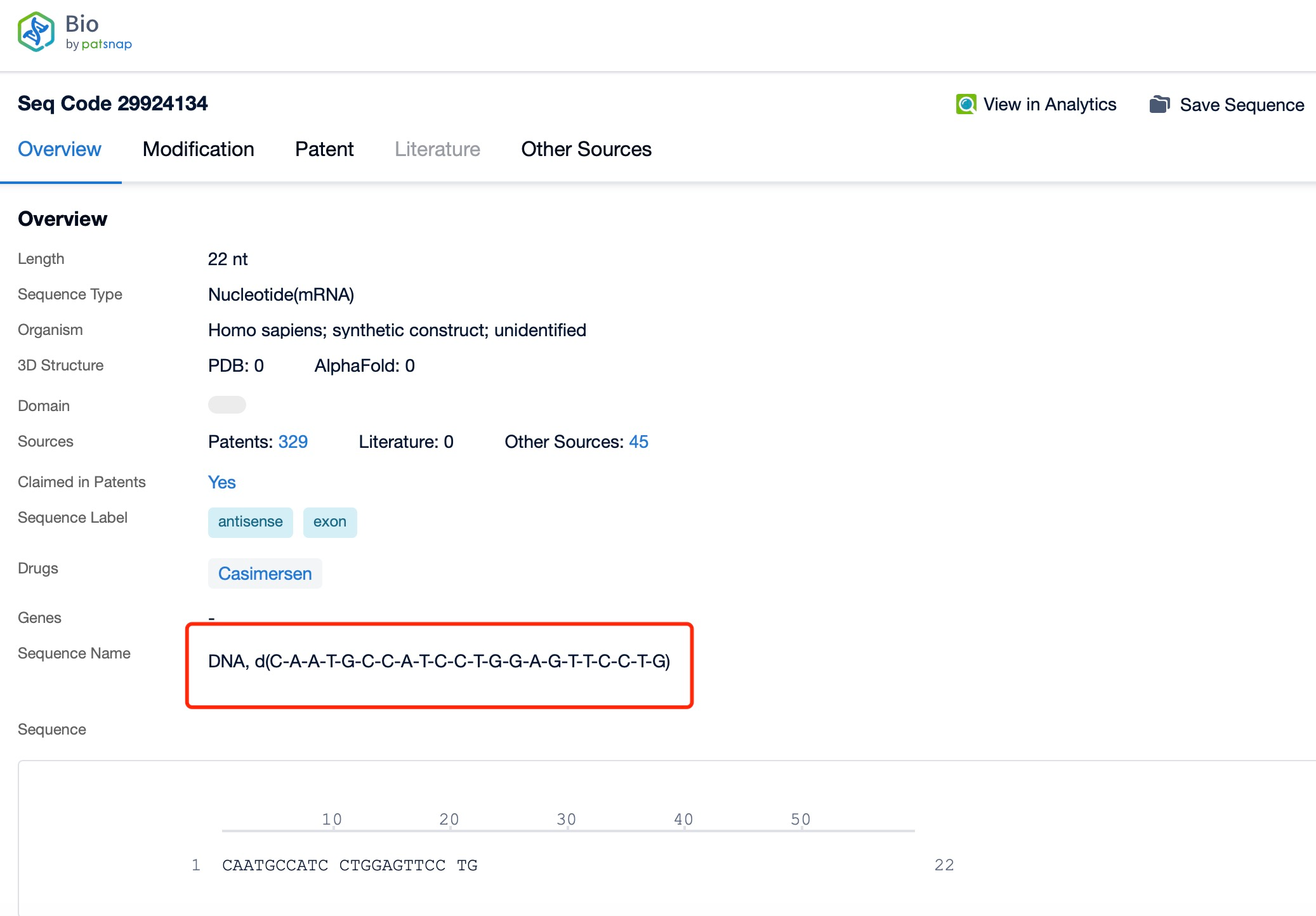
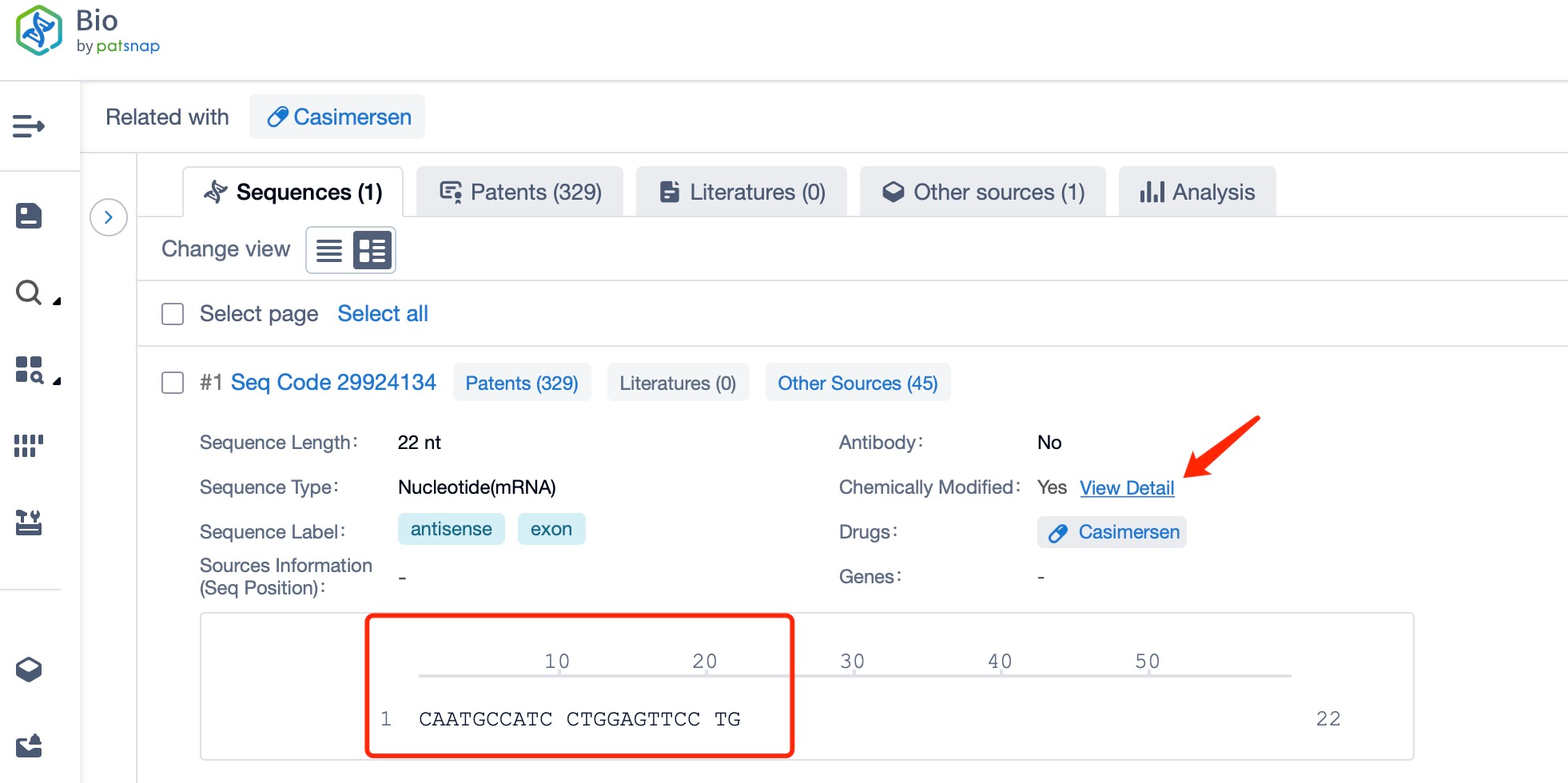
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.