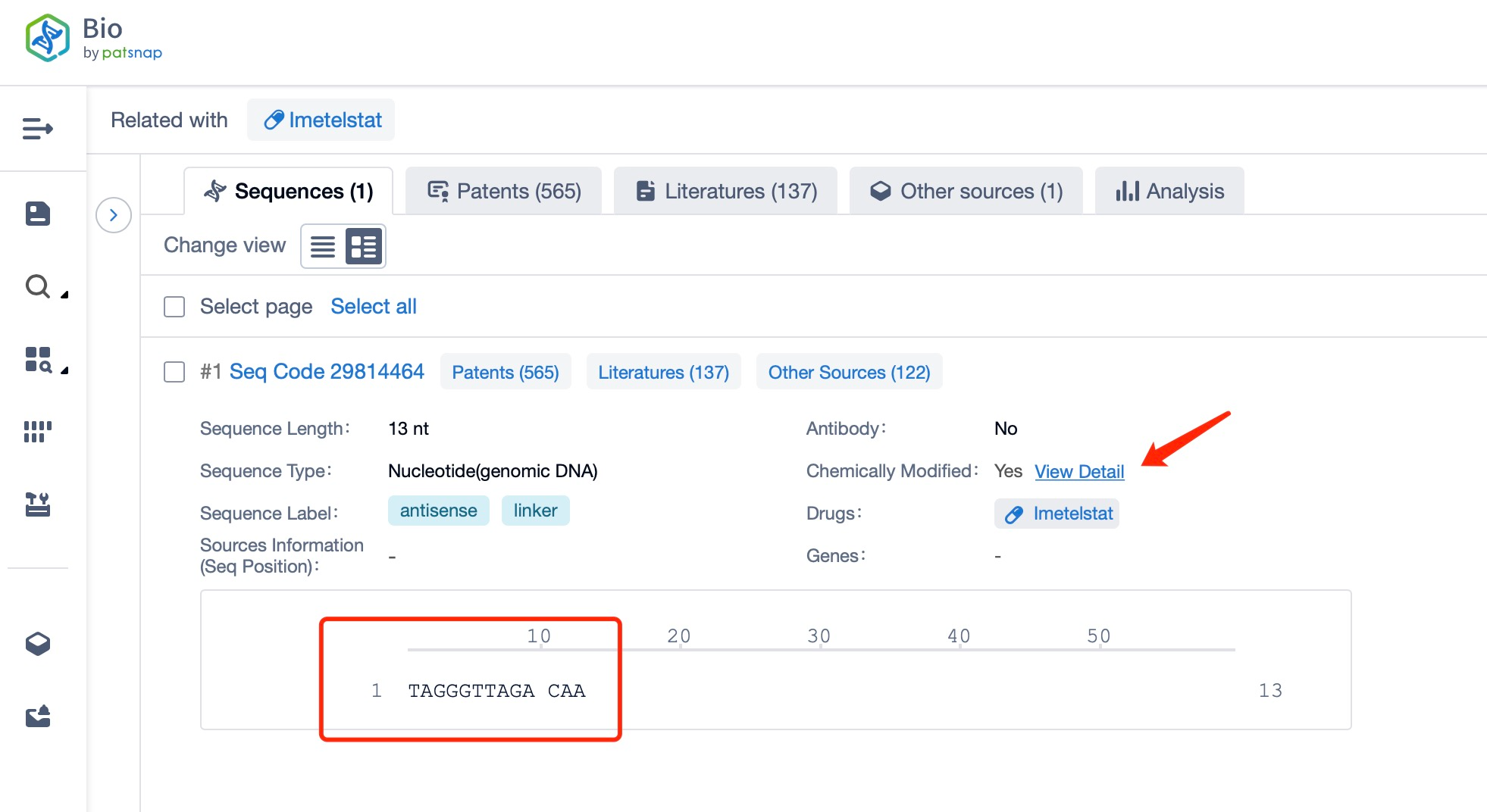
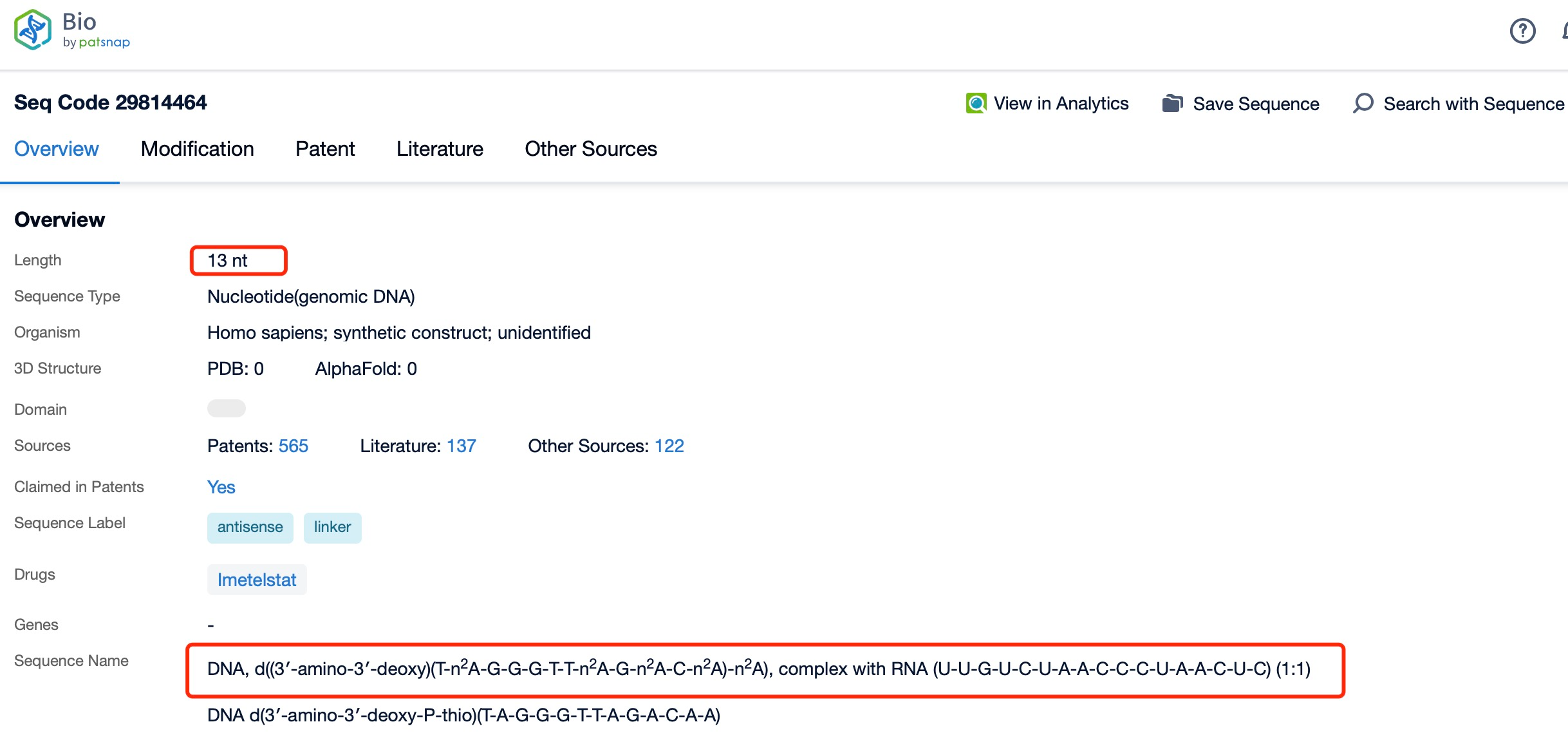
How to find the sequence of Imetelstat?
Imetelstat is a first-in-class telomerase inhibitor developed by Geron Corporation, targeting the enzyme telomerase. Telomerase plays a crucial role in maintaining the length of telomeres, which are protective structures at the ends of chromosomes. By inhibiting telomerase, Imetelstat aims to prevent cancer cells from replicating indefinitely, thereby slowing or stopping tumor growth. The drug is primarily indicated for the treatment of myelofibrosis and other hematologic malignancies.
Summary of Research Progress of Imetelstat
Imetelstat has shown promising results in preclinical and clinical studies, demonstrating its potential as a novel therapeutic approach for cancers driven by telomerase activity. The mechanism of action of Imetelstat involves binding to the RNA template of telomerase, which disrupts the enzyme's ability to add repetitive DNA sequences to the ends of chromosomes. This disruption leads to telomere shortening, which can trigger cell death in cancer cells that rely on telomerase for their survival.
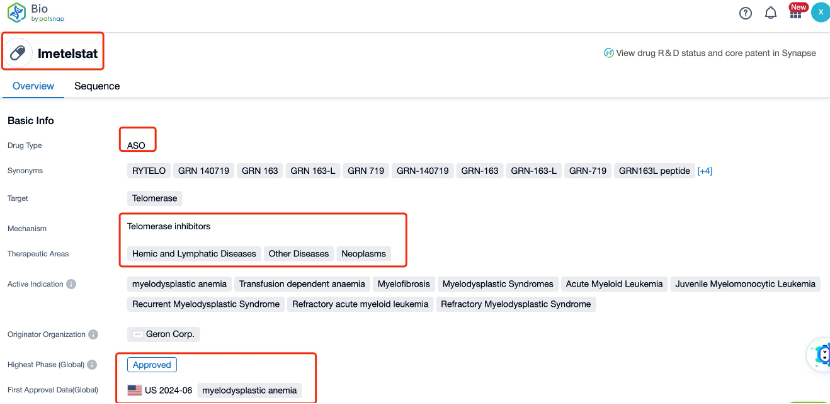
Globally, Imetelstat is still under investigation and has not yet been approved for commercial use. However, it has received orphan drug designation from both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), recognizing its potential in treating rare diseases. The global competition in the telomerase inhibitor market is limited, with few other drugs in development. This provides Imetelstat with a unique position in the field, potentially offering a new treatment option for patients with myelofibrosis and other hematologic malignancies.
Clinical research on Imetelstat has progressed through several phases. Early-phase trials have demonstrated its safety and tolerability, with dose-dependent telomere shortening observed in treated patients. Phase II trials have shown encouraging results, particularly in reducing spleen volume and improving symptoms in patients with myelofibrosis. These findings have led to the initiation of Phase III trials, which are currently ongoing to further evaluate the efficacy and safety of Imetelstat in a larger patient population.
Sequence Characteristics of Imetelstat
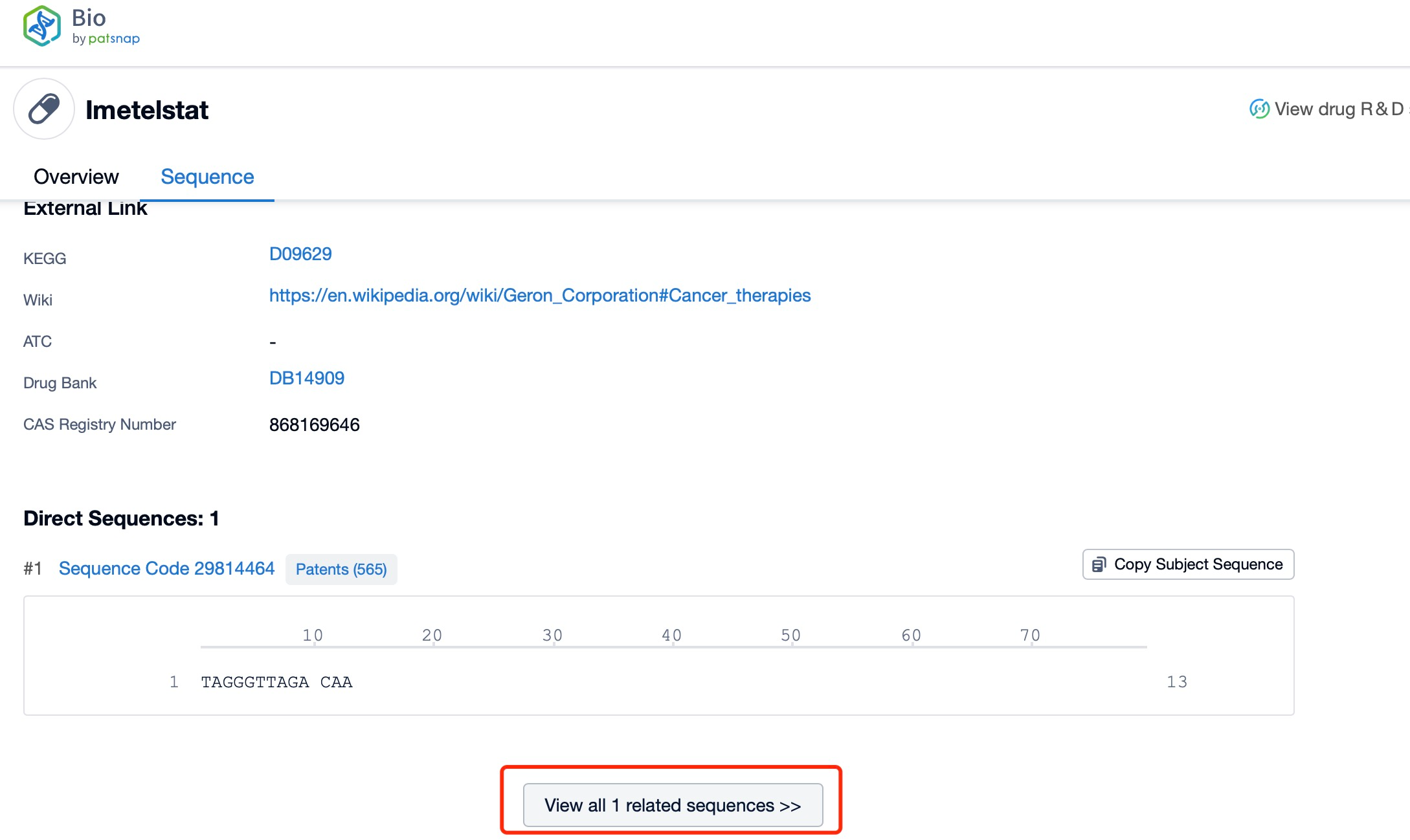
The sequence characteristics of Imetelstat are critical to its function as a telomerase inhibitor. Imetelstat is a 13-mer oligonucleotide with a specific sequence that targets the RNA template of telomerase. The sequence is designed to bind to the active site of the enzyme, thereby blocking its catalytic activity. This precise targeting ensures that Imetelstat selectively inhibits telomerase without affecting other cellular processes.
Chemical Modification and Species of Imetelstat
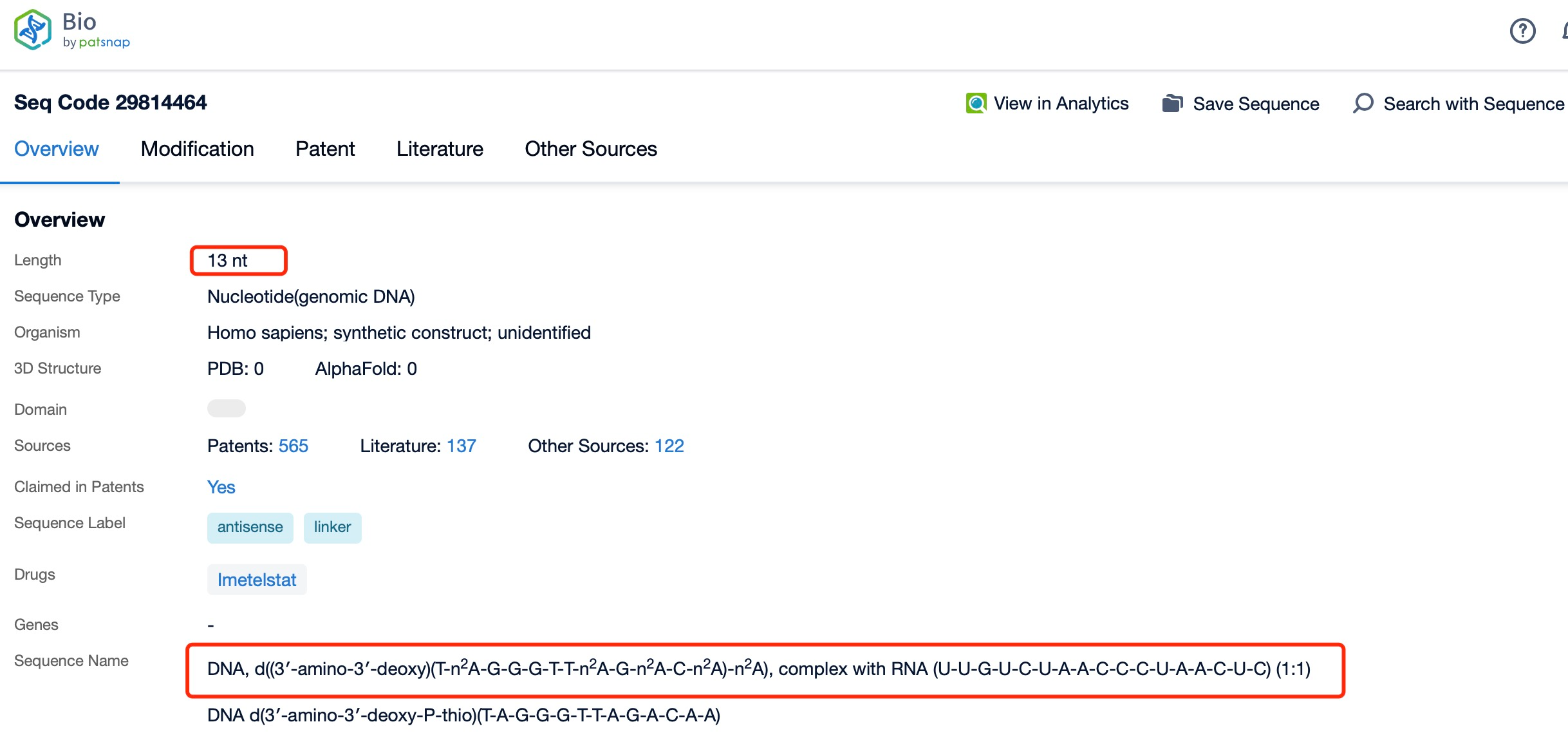
Chemical modifications play a vital role in enhancing the stability and pharmacokinetic properties of Imetelstat. The drug undergoes several chemical modifications, including the incorporation of phosphorothioate linkages and 2'-O-methylation. Phosphorothioate linkages increase the resistance of the oligonucleotide to degradation by nucleases, thereby extending its half-life in vivo. 2'-O-methylation enhances the binding affinity of Imetelstat to its target RNA, improving its potency and selectivity.
The characteristics of these chemical modifications are essential for the effectiveness of Imetelstat. Phosphorothioate linkages provide additional sulfur atoms in the backbone of the oligonucleotide, making it more resistant to enzymatic degradation. This increased stability allows Imetelstat to remain active in the bloodstream for a longer period, ensuring sustained inhibition of telomerase. 2'-O-methylation, on the other hand, introduces methyl groups at the 2' position of the ribose sugar, which increases the affinity of the oligonucleotide for its target RNA. This enhanced binding affinity translates to better target engagement and a more potent inhibitory effect.
The role of these chemical modifications is multifaceted. They not only improve the stability and pharmacokinetic properties of Imetelstat but also enhance its therapeutic index. By increasing the drug's resistance to degradation and improving its binding affinity, these modifications ensure that Imetelstat can effectively inhibit telomerase in cancer cells while minimizing off-target effects. This balance between efficacy and safety is crucial for the successful development and clinical application of Imetelstat.
Summary and Prospect
In summary, Imetelstat represents a promising advancement in the treatment of myelofibrosis and other hematologic malignancies. Its unique mechanism of action, targeting the RNA template of telomerase, sets it apart from other therapies and positions it as a potential breakthrough in the field. The ongoing clinical trials will provide further insights into its efficacy and safety, and if successful, Imetelstat could offer new hope to patients with limited treatment options. The chemical modifications that enhance its stability and binding affinity are critical to its success, ensuring that it can effectively inhibit telomerase and achieve its therapeutic goals. As research continues, Imetelstat holds the potential to transform the landscape of cancer treatment, particularly for diseases driven by telomerase activity.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
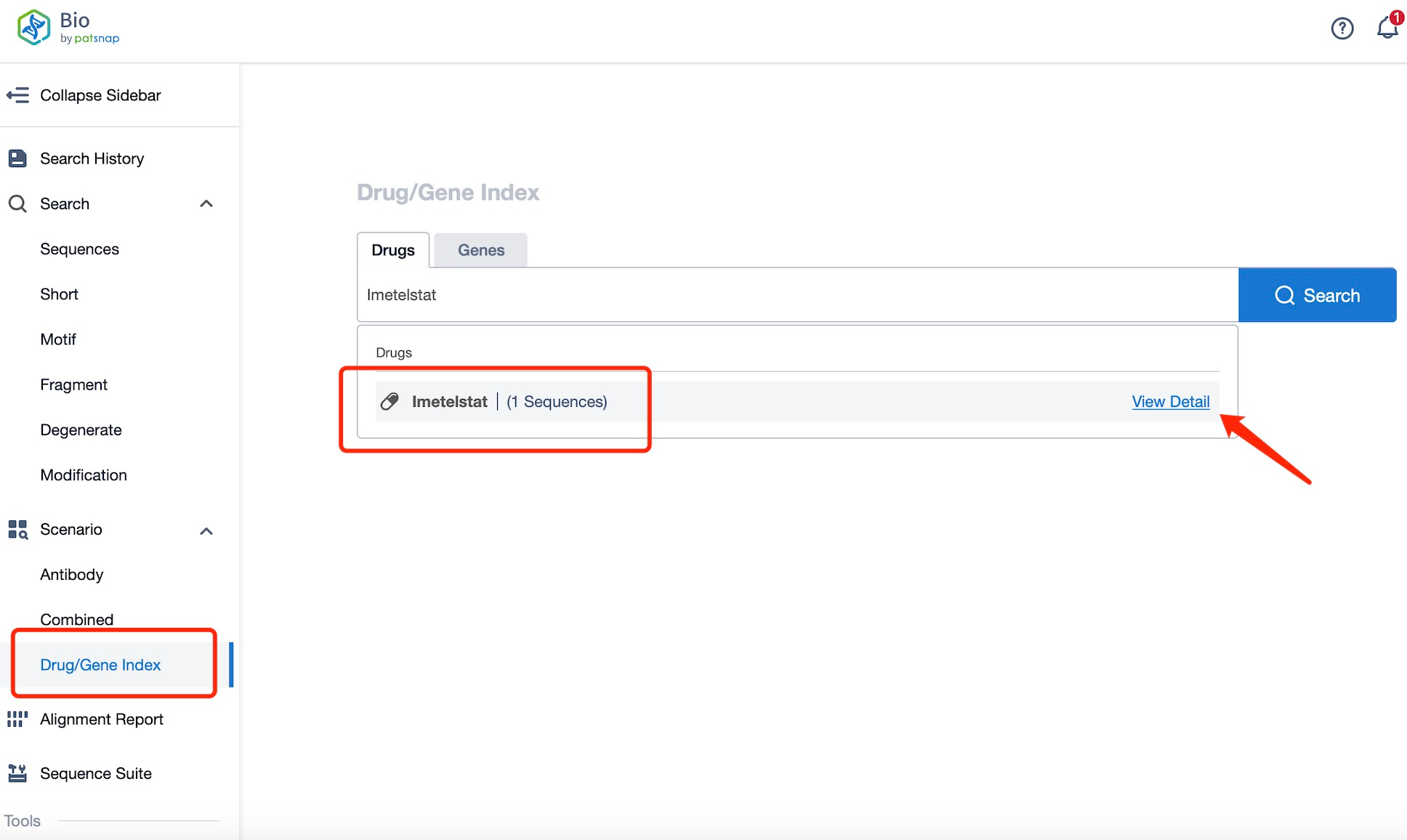
Taking defibrotide as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' defibrotide ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of defibrotide.
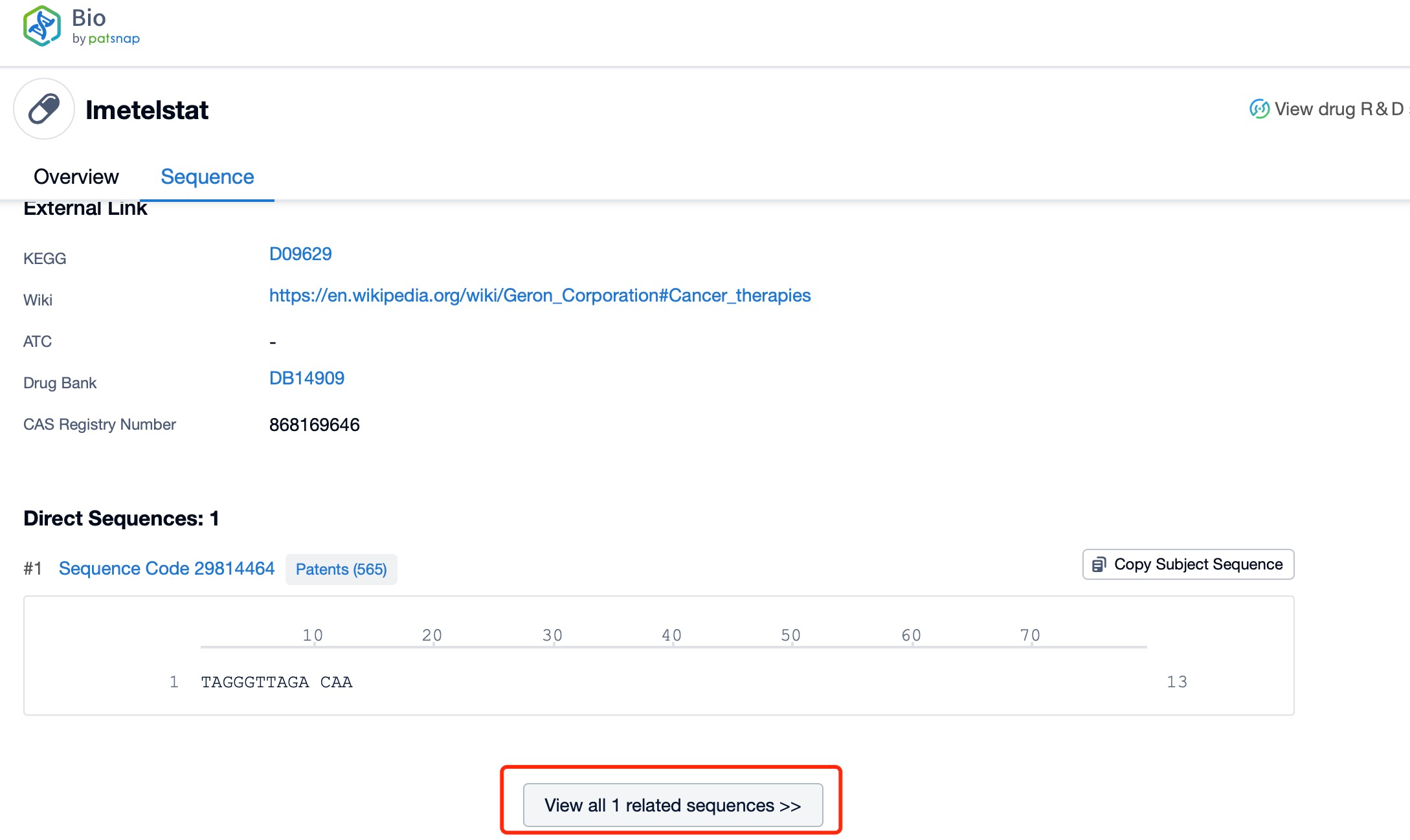
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.