PF-07328948: A Potential Breakthrough in Treating Heart Failure with Normal Ejection Fraction
PF-07328948 is a small molecule drug developed by Pfizer Inc. The drug targets BCKDK and falls within the therapeutic area of cardiovascular diseases, with a focus on treating heart failure with normal ejection fraction. As of the latest available information, PF-07328948 has reached the highest global phase of Phase 1 in its development process.
The drug's focus on heart failure with normal ejection fraction addresses a specific and challenging aspect of cardiovascular health. Heart failure with normal ejection fraction occurs when the heart is unable to relax and fill with enough blood during the resting phase. This condition represents a significant portion of heart failure cases and is associated with substantial morbidity and mortality.
PF-07328948's target of BCKDK suggests a potential new approach to treating heart failure and may offer a novel therapeutic avenue within the cardiovascular disease landscape. BCKDK is involved in the regulation of branched-chain amino acid metabolism and has implications for metabolic homeostasis, potentially impacting cardiovascular health.
Below, we will use the drug PF-07328948 as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
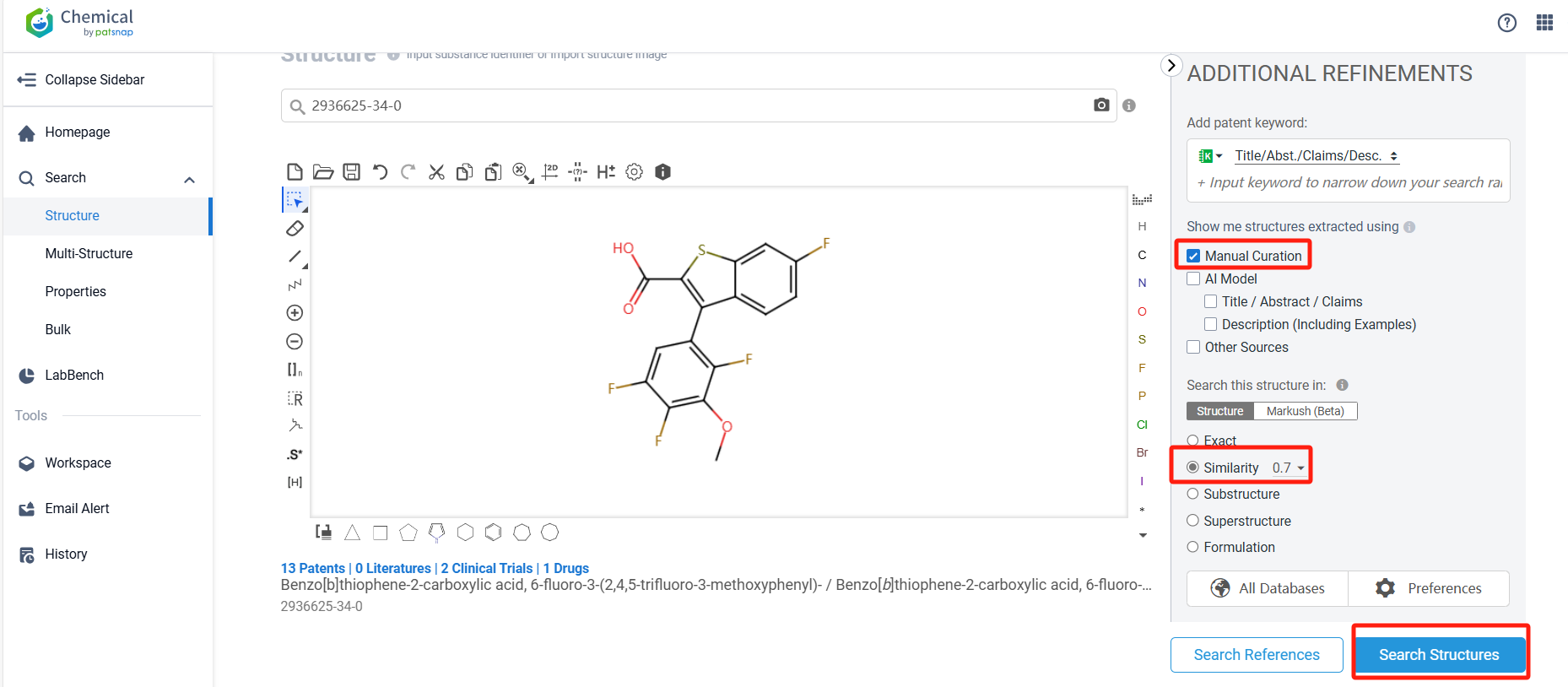
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of PF-07328948 (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find the innovative drug PF-07328948, as disclosed in the patent application with the publication number US20230167080A1, first made public on 2023-06-01.
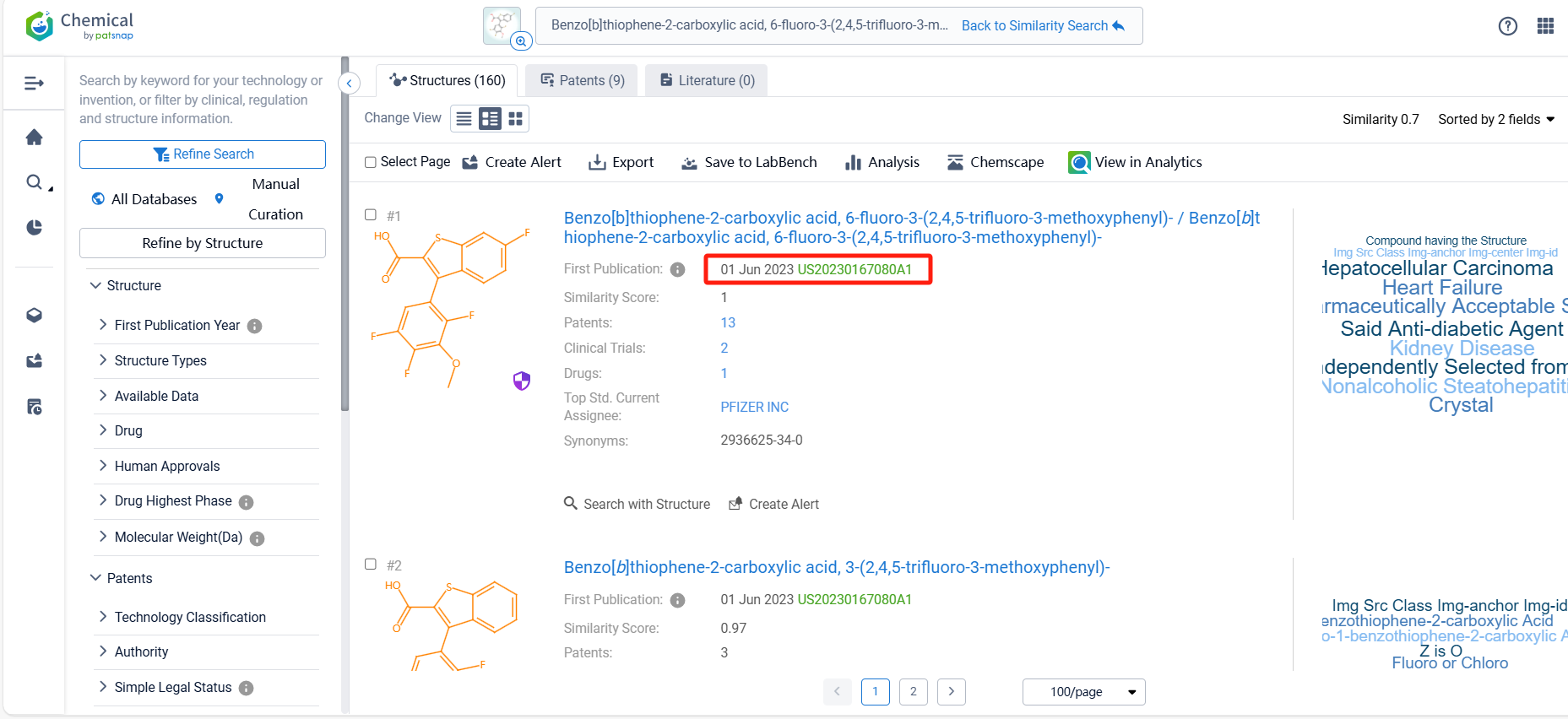
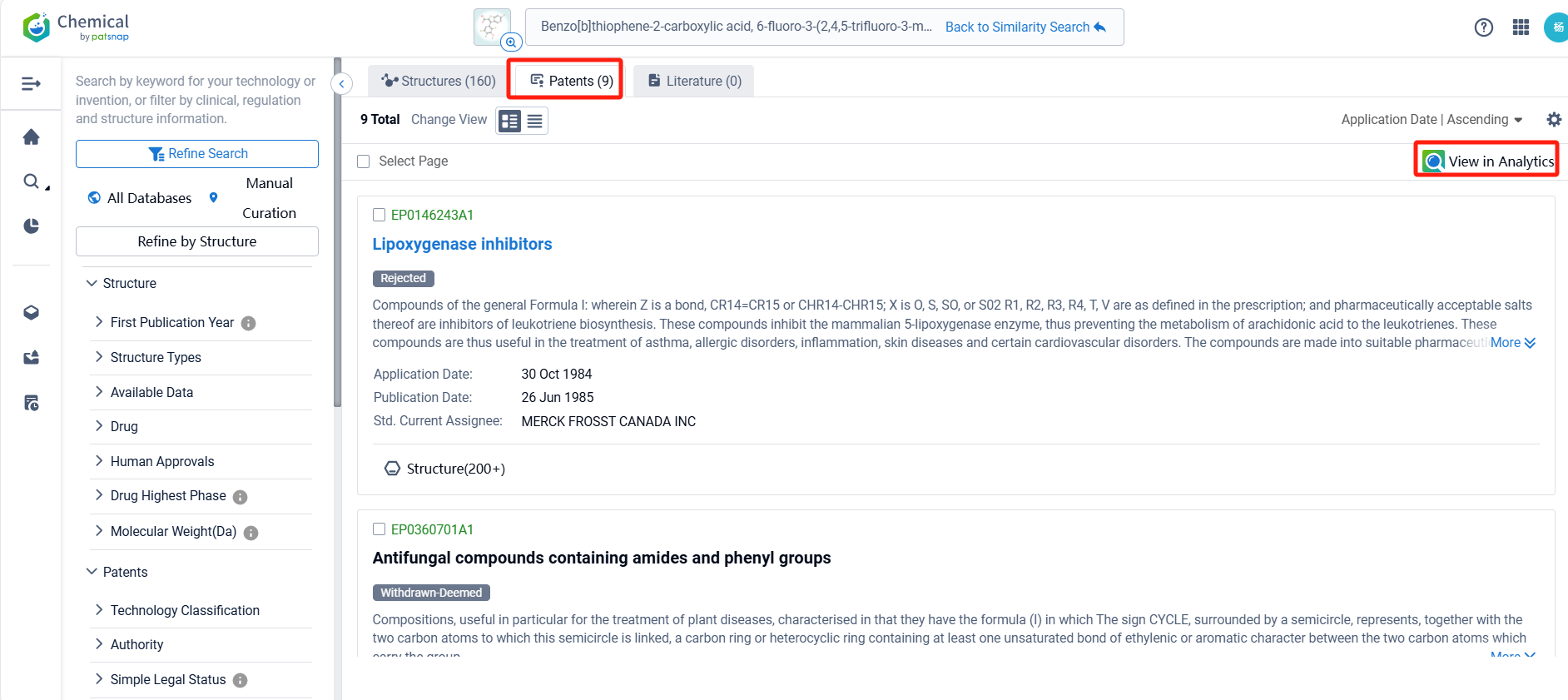
There are 9 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
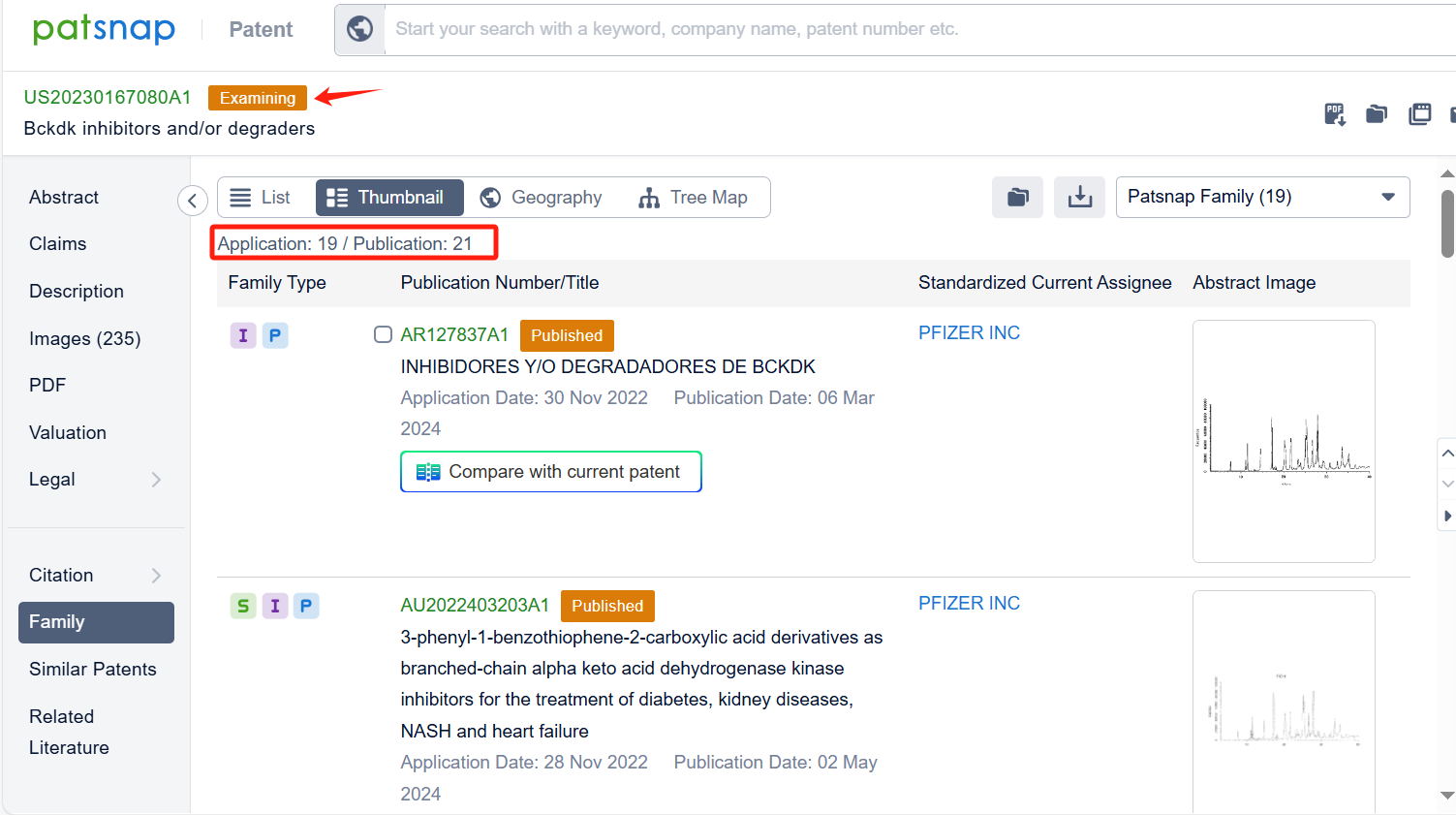
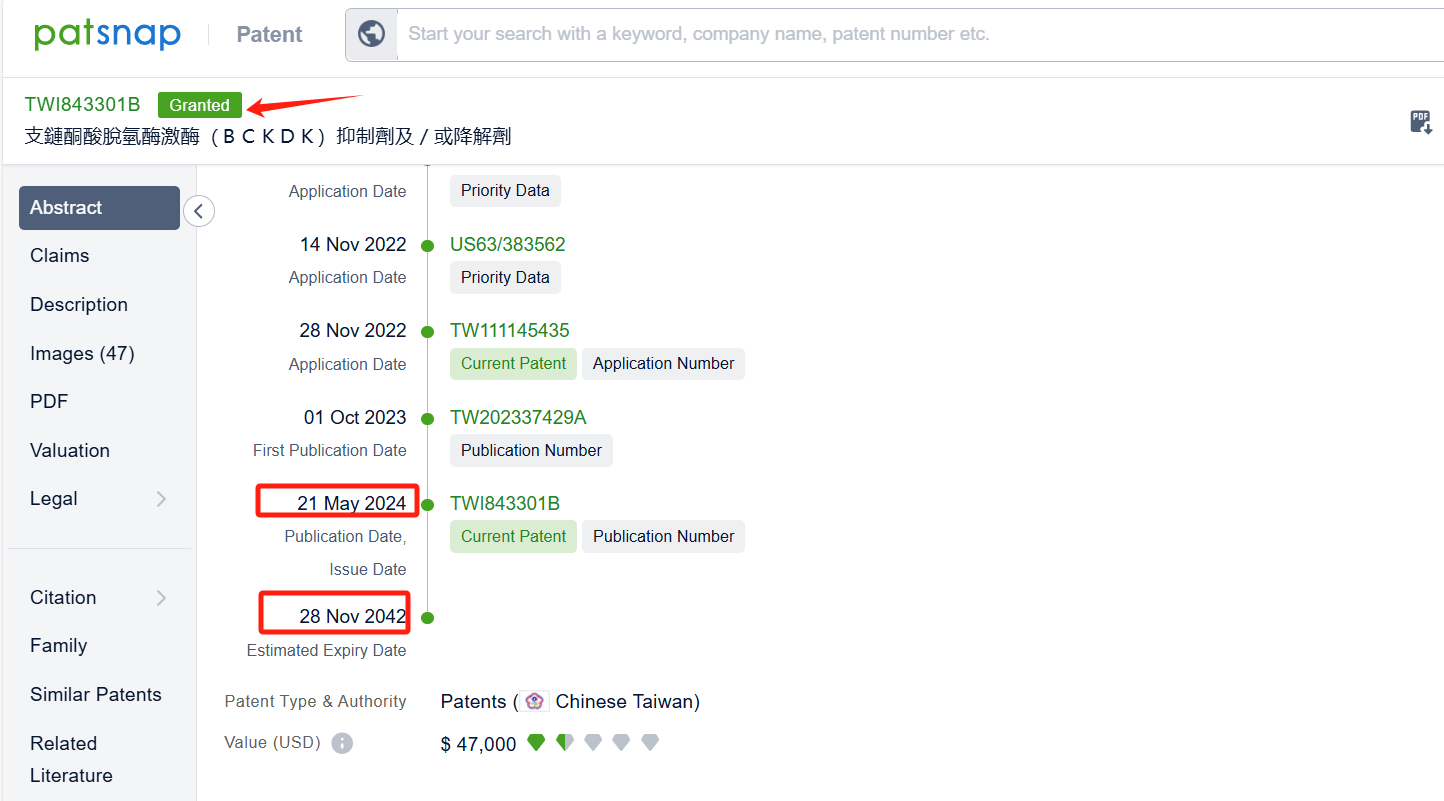
By reviewing the aforementioned patents, we can observe that the aforementioned PCT international application has entered the United States, China, South Korea, Japan, and other countries. Currently, the Taiwan patent TWI843301B was granted on May 21, 2024, while patent applications in other countries are still under substantive examination. Upon reviewing the aforementioned 9 patents, it appears that no other companies have filed fast follow patents on Pfizer Inc.
As PF-07328948 continues through the drug development process, further studies and clinical trials will be necessary to assess its effectiveness and safety profile. If successful, this small molecule drug could offer a valuable addition to the treatment options available for individuals experiencing heart failure with normal ejection fraction, potentially improving patient outcomes in this challenging therapeutic area.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.