KOSELUGO® (Selumetinib) Shows Significant Improvement in Response Rate Compared to Placebo
Alexion, AstraZeneca Rare Disease, and Merck (NYSE: MRK), referred to as MSD outside the U.S. and Canada, have today revealed favorable topline outcomes from the Phase 3 KOMET study. This represents the largest global randomized double-blind placebo-controlled multicenter Phase 3 investigation involving adults diagnosed with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN). The topline data indicated that KOSELUGO, which is an oral selective MEK inhibitor, achieved a statistically significant and clinically meaningful enhancement in the objective response rate (ORR), the primary endpoint of the study, compared to placebo in this group of adult patients.
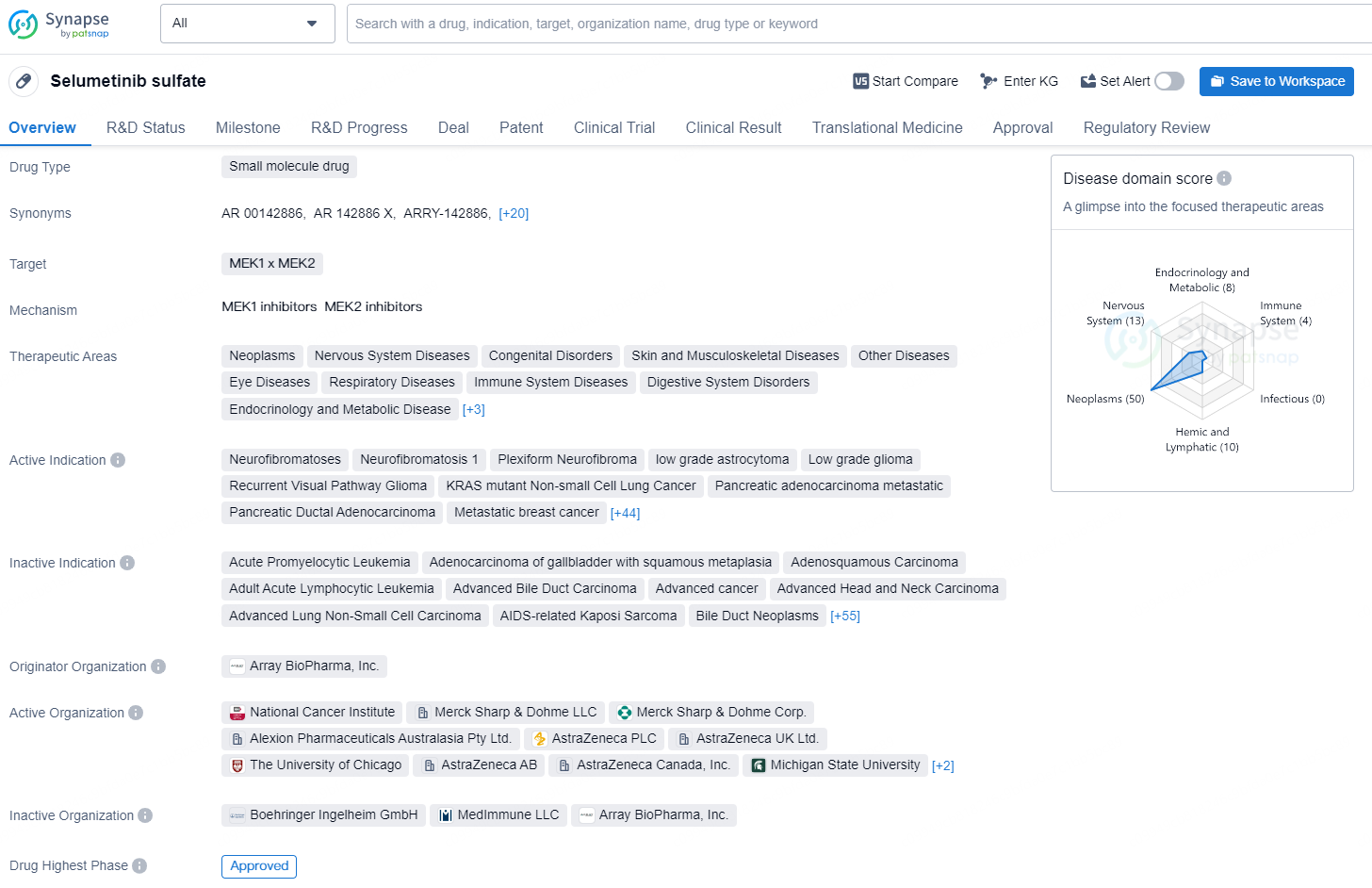
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Neurofibromatosis type 1 (NF1) is a rare and progressive genetic disorder that impacts around 1.7 million individuals globally, with approximately 70% being adults. Tumors can form on nerve sheaths in 30-50% of patients, potentially leading to severe symptoms. NF1 is typically identified in early childhood, but the condition can continue to advance into adulthood. Currently, there are no approved treatments for adult patients, resulting in many facing disfigurement, functional challenges, chronic pain, or the need for multiple surgical interventions.
Professor Ignacio Blanco Guillermo, M.D., Ph.D., the head of genetic counseling and clinical genetics at Germans Trias i Pujol University Hospital, the chairman of the Spanish National Reference Center for Adult Neurofibromatosis patients, and the principal investigator of the KOMET trial, commented: "Due to the scarcity of management options for adult patients with NF1 plexiform neurofibromas, many experience significant functional limitations and symptoms that greatly affect their quality of life. The clinically relevant data indicate that KOSELUGO has the potential to improve patient care by helping to decrease the size of plexiform neurofibromas."
Marc Dunoyer, CEO of Alexion, stated: "These encouraging results indicate that KOSELUGO, the first and only approved targeted therapy for certain children with NF1 plexiform neurofibromas, may also be beneficial for adult patients who currently have no approved targeted treatment options. As the largest global placebo-controlled Phase 3 trial for adults with NF1 plexiform neurofibromas, KOMET highlights our commitment to enhancing treatment alternatives for individuals afflicted by this challenging condition. We eagerly anticipate presenting these findings to regulatory bodies."
Dr. Scot Ebbinghaus, Vice President of Global Clinical Development at Merck Research Laboratories, noted: "Adults suffering from NF1 require immediate treatment alternatives to address symptomatic, inoperable plexiform neurofibromas. The positive outcomes from the Phase 3 KOMET trial underscore the possibility of extending the use of KOSELUGO from pediatric to adult patients facing this rare and complex genetic disorder."
In the trial, the overall response rate (ORR) was measured as the percentage of participants exhibiting a confirmed complete response (complete disappearance of plexiform neurofibromas) or a partial response (at least a 20% decrease in tumor volume) by the 16th cycle (28 days per cycle), as evaluated by independent central review (ICR) in accordance with the response evaluation criteria for neurofibromatosis and schwannomatosis (REiNS).
The safety profile of KOSELUGO observed in this study aligned with findings from previous clinical trials in children and adolescents, with no new safety concerns identified. Alexion, part of AstraZeneca Rare Disease, plans to share these results with regulatory authorities and will present them at an upcoming medical conference. AstraZeneca and Merck are collaboratively developing and commercializing KOSELUGO on a global scale.
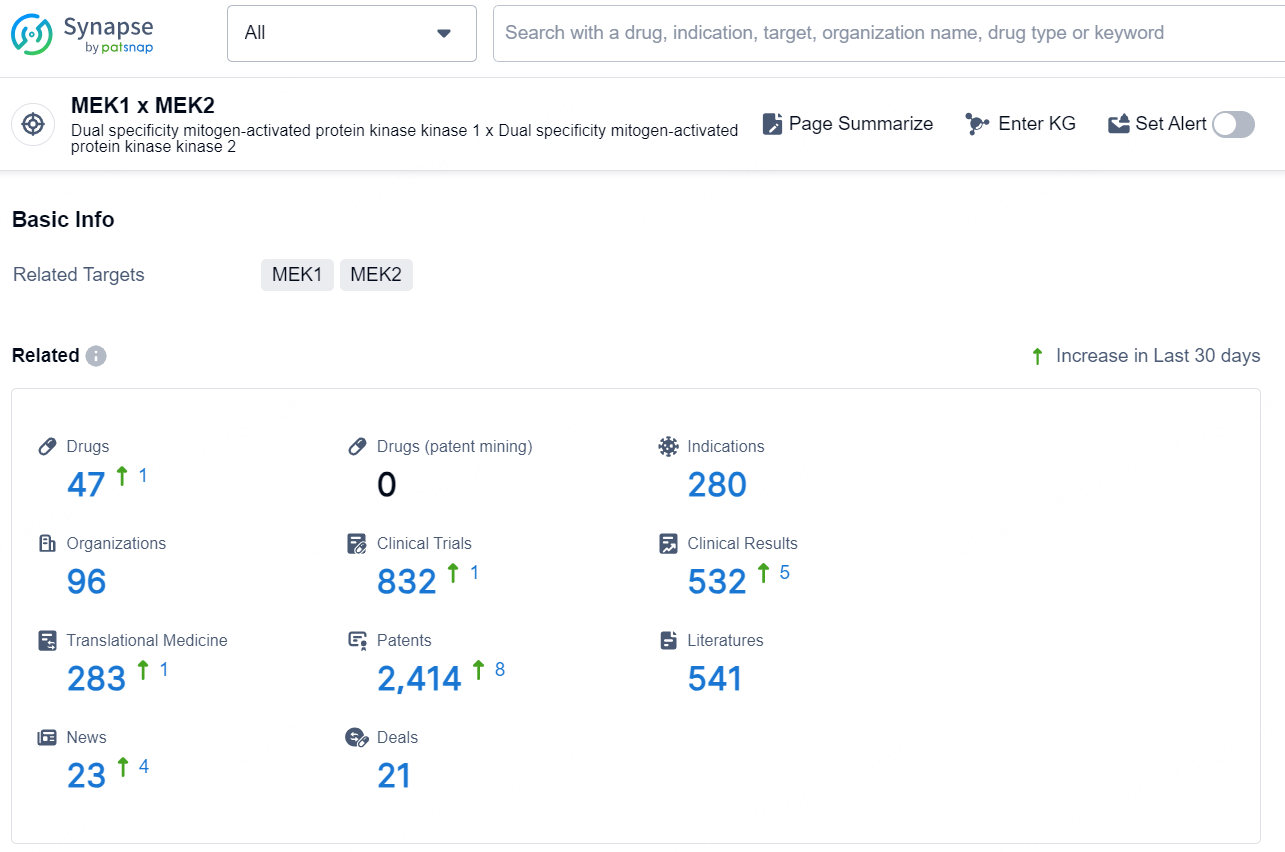
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 13, 2024, there are 47 investigational drug for the MEK1 and MEK2 target, including 280 indications, 96 R&D institutions involved, with related clinical trials reaching 832, and as many as 2414 patents.
Selumetinib sulfate is a small molecule drug that targets MEK1 and MEK2. It is used in the treatment of a wide range of therapeutic areas, including neoplasms, nervous system diseases, congenital disorders, skin and musculoskeletal diseases, eye diseases, respiratory diseases, immune system diseases, digestive system disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, otorhinolaryngologic diseases, and urogenital diseases.