How to find the sequence of Mipomersen?
Mipomersen, developed by Isis Pharmaceuticals (now known as Ionis Pharmaceuticals) and marketed by Genzyme (a Sanofi company), is an antisense oligonucleotide that targets the apolipoprotein B-100 (ApoB-100) mRNA. ApoB-100 is a key component of low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) particles, which are major contributors to atherosclerosis and cardiovascular disease. Mipomersen is indicated for the treatment of homozygous familial hypercholesterolemia (HoFH), a rare and severe genetic disorder characterized by extremely high levels of LDL cholesterol. By targeting the ApoB-100 mRNA, mipomersen aims to reduce the production of ApoB-100, thereby lowering LDL cholesterol levels and reducing the risk of cardiovascular events.
Summary of Research Progress of Mipomersen
Mipomersen works by binding to the ApoB-100 mRNA, leading to its degradation via RNase H-mediated cleavage. This prevents the synthesis of ApoB-100, which in turn reduces the levels of LDL and VLDL particles in the blood. By addressing the underlying genetic defect, mipomersen aims to lower cholesterol levels and improve cardiovascular outcomes in patients with HoFH. Mipomersen is administered subcutaneously once weekly, offering a convenient and less invasive route of administration compared to other lipid-lowering therapies. The drug has been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of HoFH.
Globally, the competition in the lipid-lowering market is intense, with several established and emerging therapies. Mipomersen faces competition from statins, which are the first-line treatment for hypercholesterolemia and are widely used for their effectiveness and safety. For patients with HoFH, mipomersen competes with other targeted therapies, such as lomitapide (Juxtapid) and evinacumab (Evkeeza). Lomitapide is a microsomal triglyceride transfer protein inhibitor that reduces the production of VLDL, while evinacumab is a monoclonal antibody that targets angiopoietin-like protein 3 (ANGPTL3), which is involved in lipid metabolism. Despite this competition, mipomersen offers a unique advantage with its subcutaneous administration and its ability to significantly reduce LDL cholesterol levels, especially in patients who do not respond adequately to other therapies.
Clinical research on mipomersen has demonstrated its efficacy and safety in reducing LDL cholesterol levels in patients with HoFH. The Phase 3 clinical trials, such as the APOLLO study, showed that mipomersen could significantly lower LDL cholesterol levels by up to 25% in treated patients. The drug was generally well-tolerated, with common side effects including injection site reactions, flu-like symptoms, and liver enzyme elevations. Ongoing and future trials aim to further evaluate the long-term safety and efficacy of mipomersen, as well as explore its potential in other forms of severe hypercholesterolemia.
Sequence Characteristics of Mipomersen
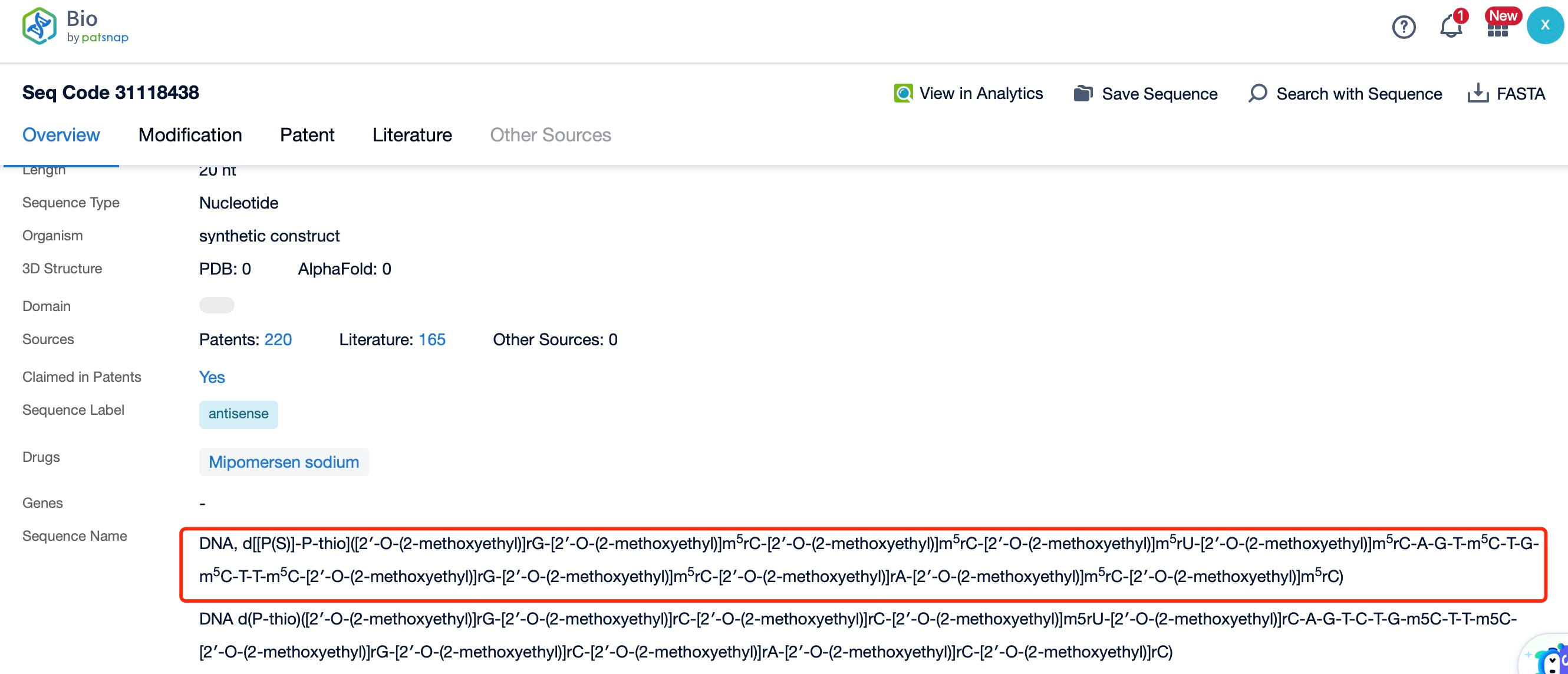
Mipomersen is a 20-nucleotide phosphorothioate-modified oligonucleotide. The sequence of mipomersen is specifically designed to bind to the ApoB-100 mRNA, targeting a region that is essential for the synthesis of ApoB-100. This sequence is optimized to ensure high specificity and efficiency in binding to the target mRNA, which is crucial for the drug's therapeutic efficacy and safety. The precise sequence of mipomersen is a proprietary information, but it is known to be highly complementary to the target ApoB-100 mRNA, allowing for effective RNase H-mediated cleavage and degradation.
Chemical Modification and Species of Mipomersen
The chemical modifications in mipomersen include phosphorothioate linkages. The phosphorothioate backbone replaces one of the non-bridging oxygen atoms in the phosphate group with a sulfur atom, enhancing the stability of the oligonucleotide and protecting it from nuclease degradation. This modification is crucial for the drug's ability to reach and bind to the target mRNA in the liver. The phosphorothioate backbone also improves the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are important for its therapeutic effectiveness.
The phosphorothioate modifications in mipomersen provide several key advantages. First, they significantly enhance the stability of the oligonucleotide, allowing it to remain active in the cellular environment for a longer period. This increased stability is crucial for the drug's effectiveness in binding to and degrading the ApoB-100 mRNA. Second, the phosphorothioate backbone improves the pharmacokinetic profile of the drug, increasing its half-life and bioavailability. This means that the drug can be administered less frequently, which is beneficial for patient convenience and compliance. Third, the phosphorothioate modifications reduce the risk of off-target effects by enhancing the specificity of the oligonucleotide for its target mRNA.
The role of the phosphorothioate modifications in mipomersen is multifaceted. They protect the oligonucleotide from degradation by nucleases, ensuring that it can reach its target in the liver. They also enhance the binding affinity of the oligonucleotide to the ApoB-100 mRNA, ensuring efficient and specific RNase H-mediated cleavage. Additionally, the phosphorothioate modifications improve the pharmacokinetic properties of the drug, such as its half-life and tissue distribution, which are essential for its therapeutic effectiveness. These modifications also reduce the immunogenicity of the oligonucleotide, minimizing the risk of adverse immune responses.
Summary and Prospect
In summary, mipomersen represents a significant advancement in antisense technology and the treatment of homozygous familial hypercholesterolemia (HoFH). Its mechanism of action, involving the degradation of ApoB-100 mRNA, has shown promising results in reducing LDL cholesterol levels and improving cardiovascular outcomes in affected patients. Despite facing competition from other lipid-lowering therapies, mipomersen offers a unique advantage with its subcutaneous administration and its ability to significantly reduce LDL cholesterol levels, especially in patients who do not respond adequately to other therapies. Future research may focus on improving delivery methods and exploring combination therapies to enhance its clinical utility. The sequence characteristics and chemical modifications of mipomersen, including its phosphorothioate backbone, contribute to its stability, specificity, and efficacy, making it a valuable tool in the management of severe hypercholesterolemia. However, the need for careful monitoring of liver function and the management of side effects will continue to be important considerations in its clinical use.
How to find the sequence of an ASO?
In Patsnap Bio, you can find the sequence and latest research and development advances of all ASOs.
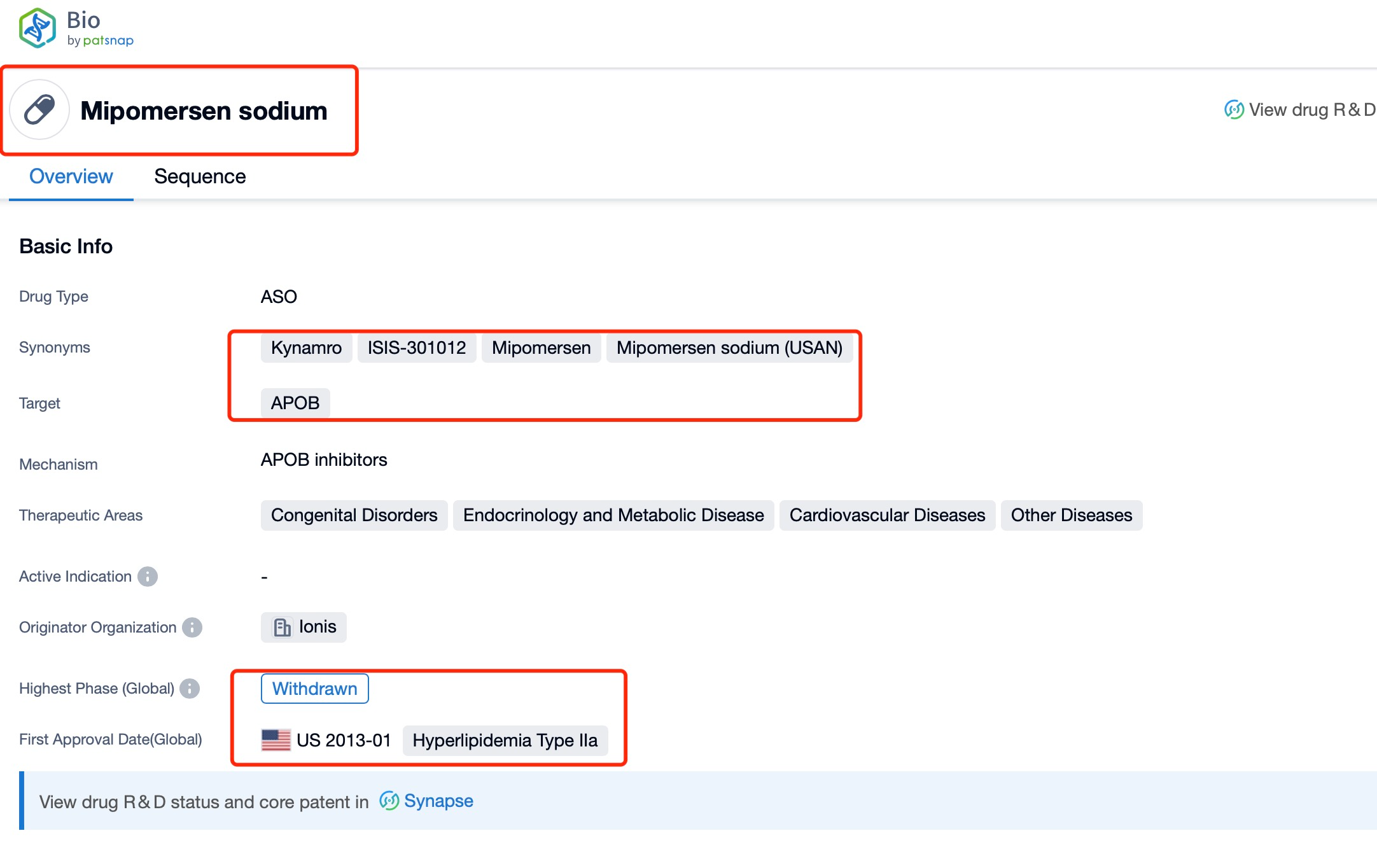
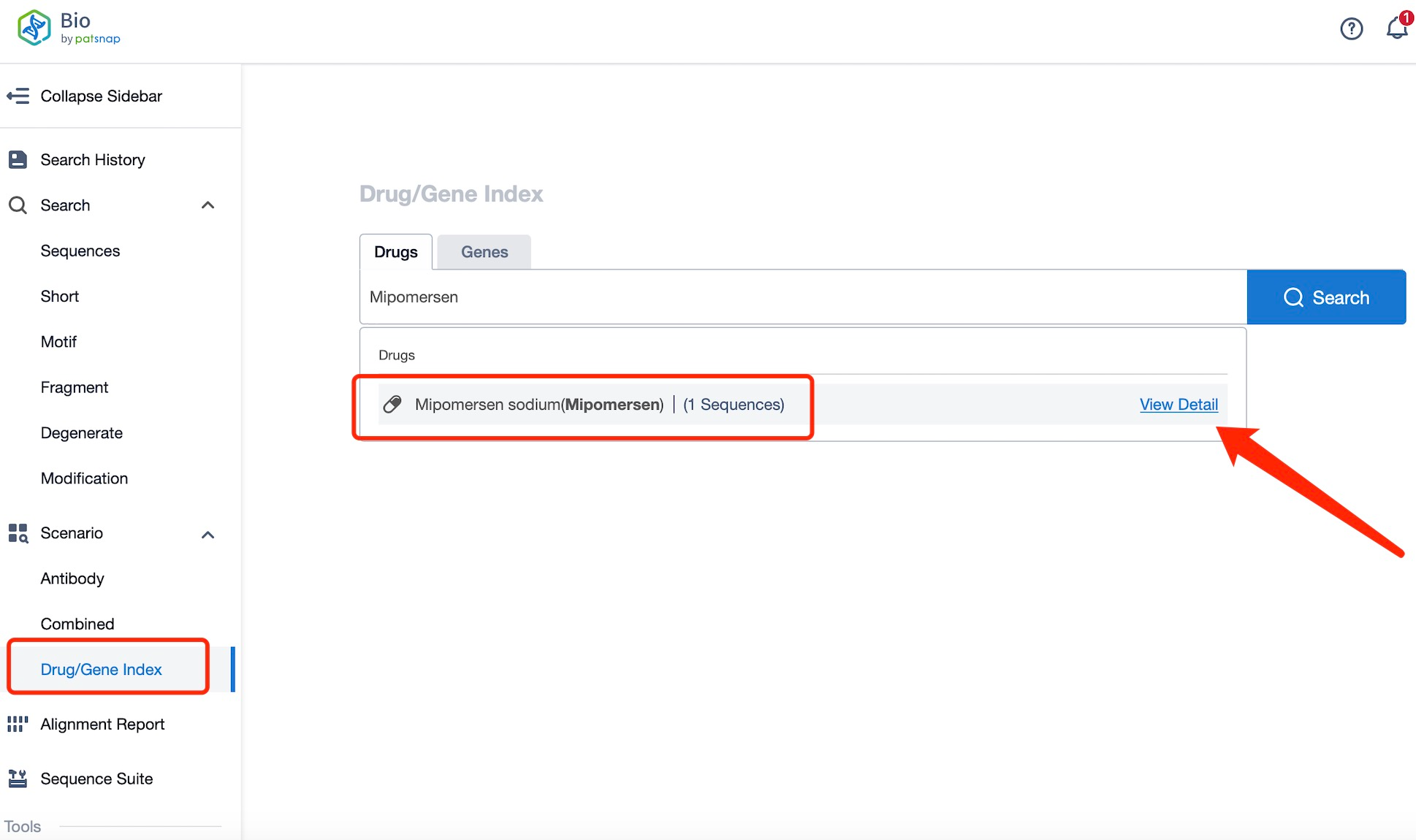
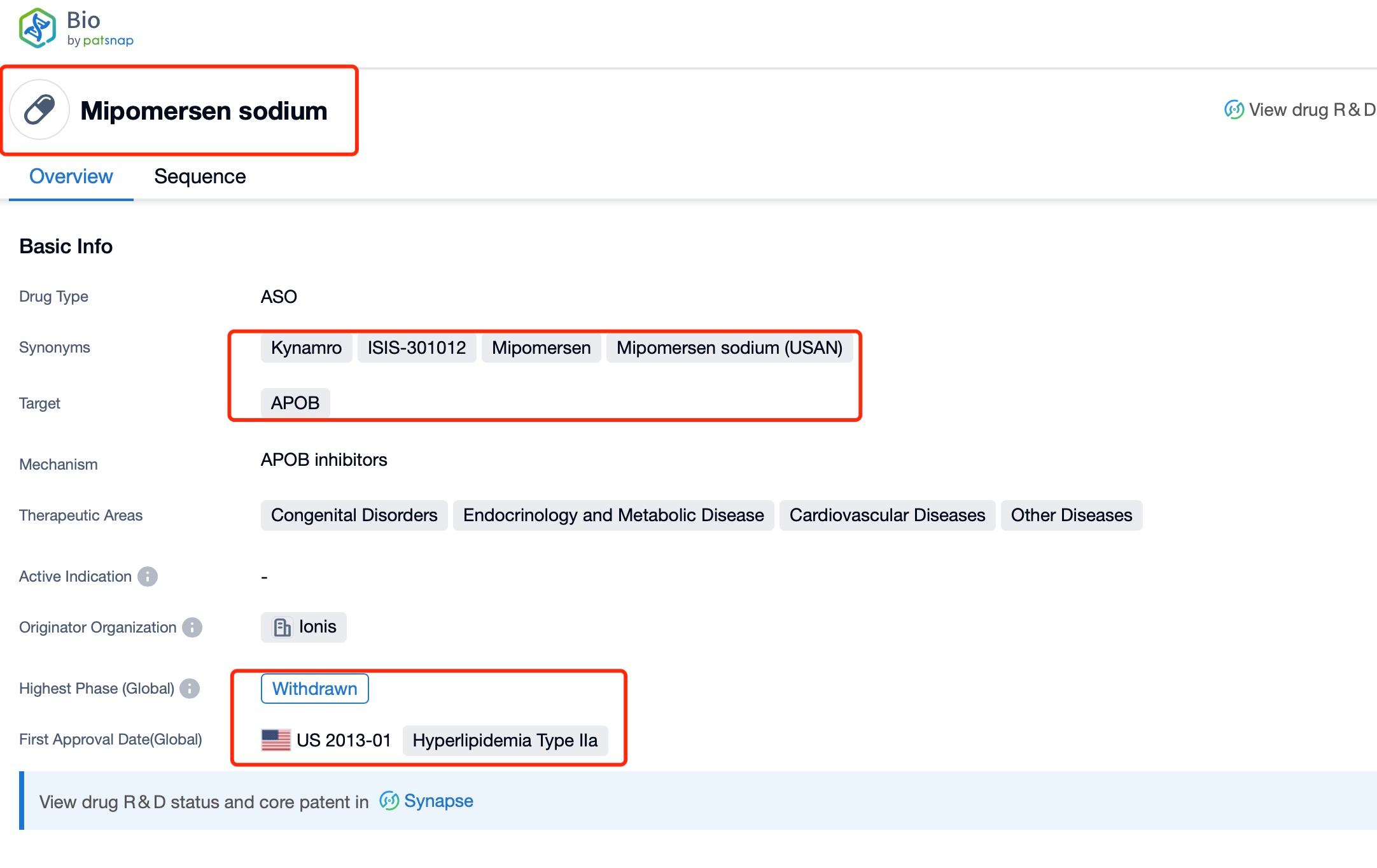
Taking Mipomersen as an example, first click on the Drug/Gene Index on the Patsnap Bio homepage. Here you can search for sequence information by drug and gene names. Enter ' Mipomersen ' in the search box and click to view the details. On the details page, you can find the basic information and research progress of Mipomersen.
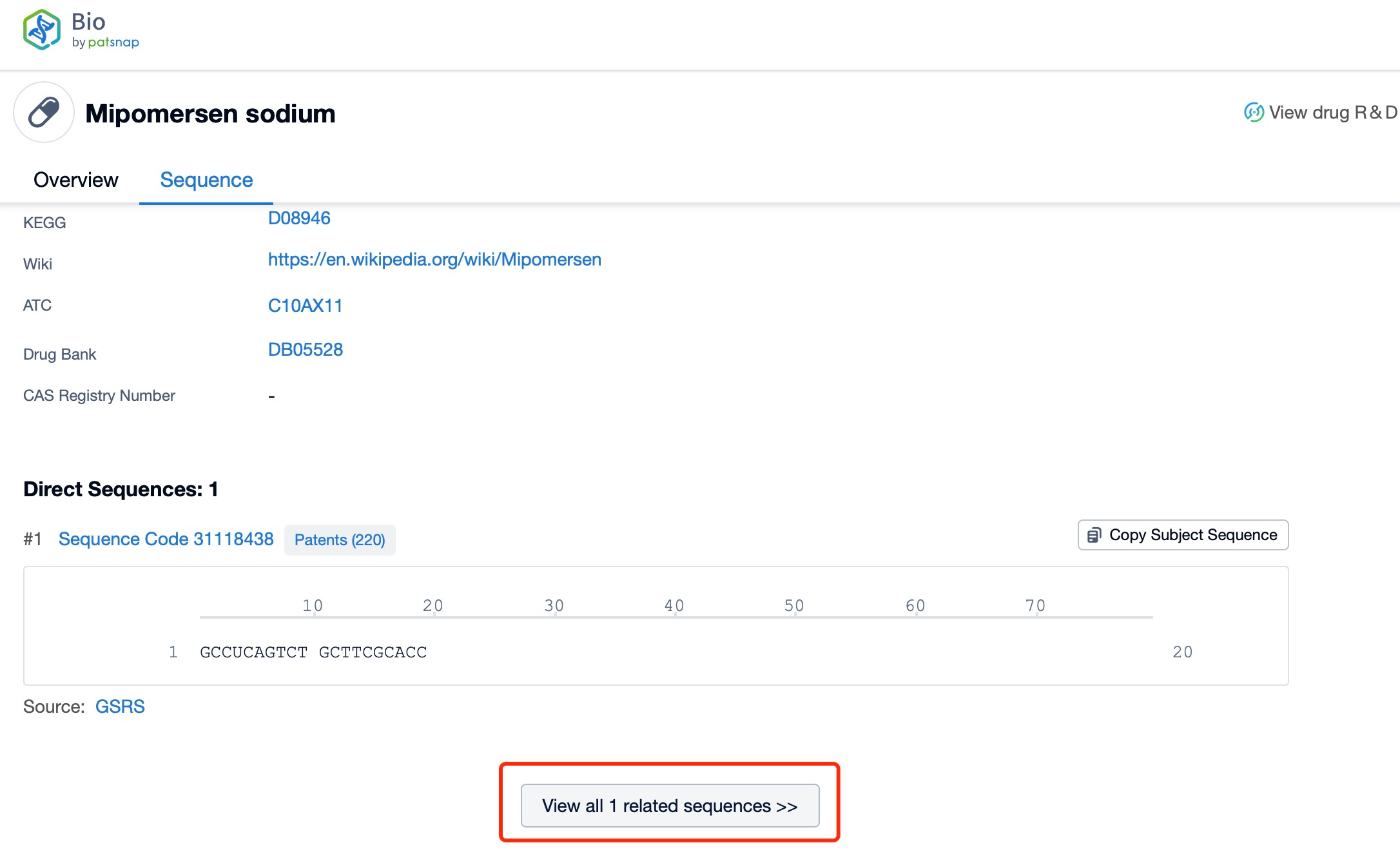
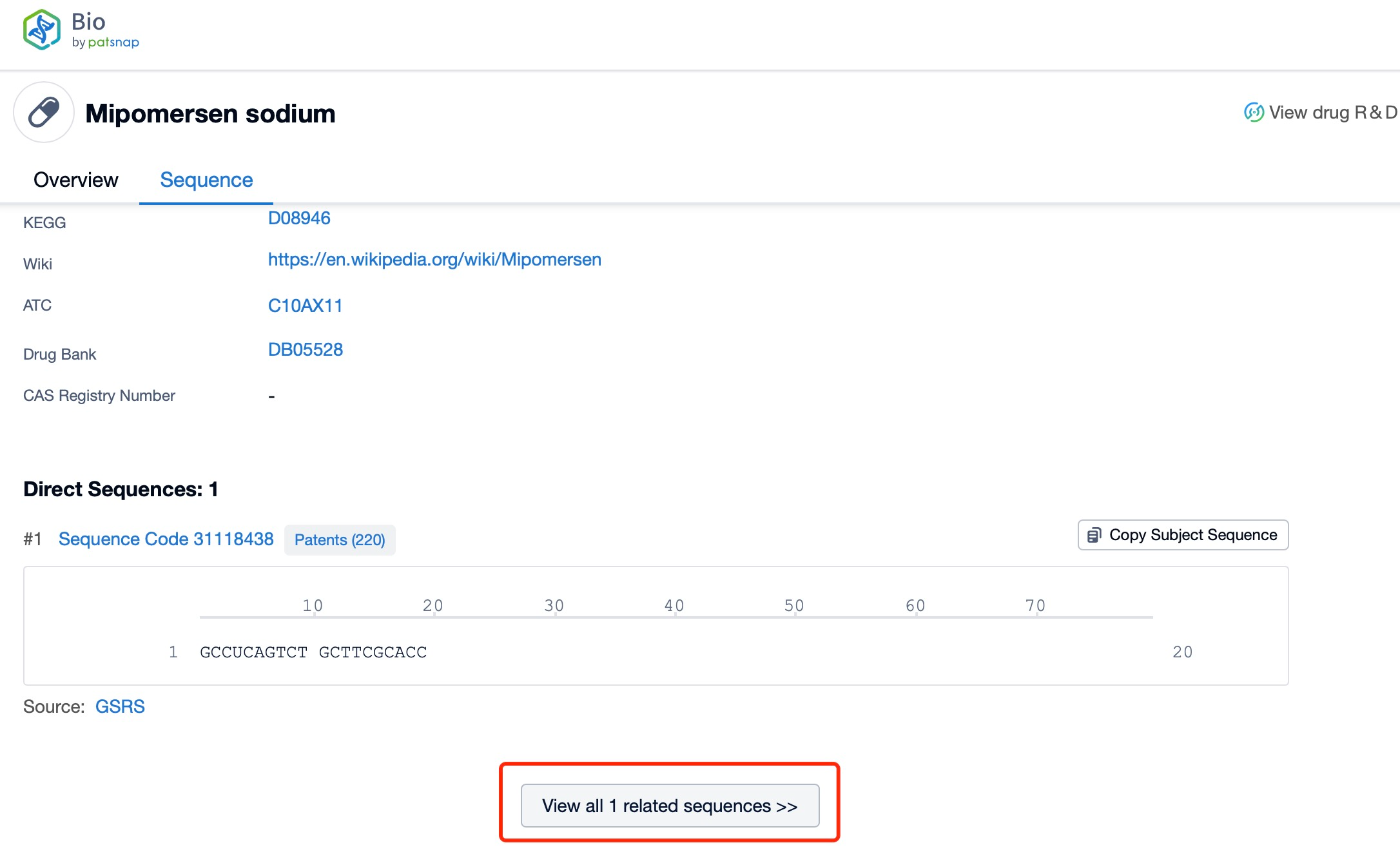
Click "View all related sequences" below the sequence information to search for and retrieve all biological sequences similar to this information.
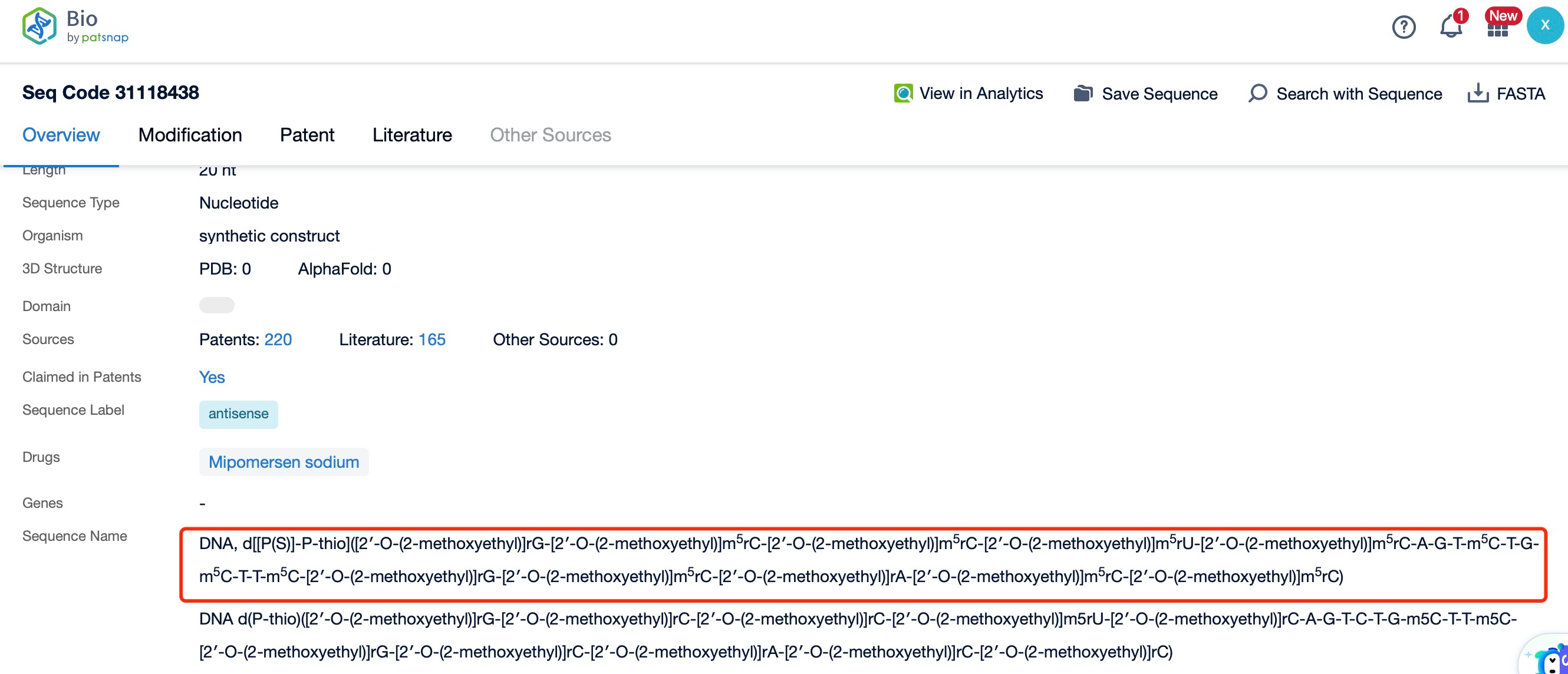
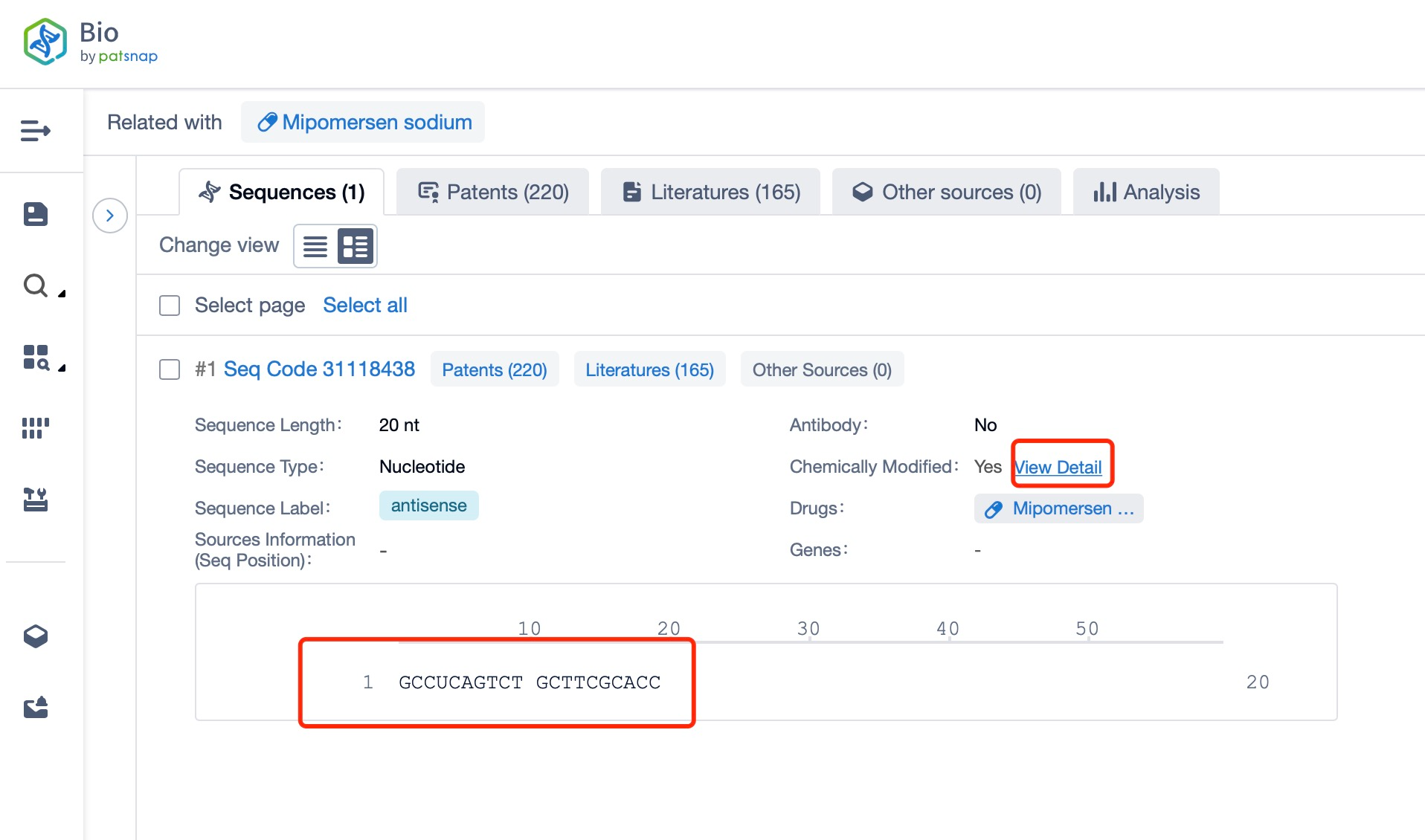
Clicking on the sequence name will provide you with all the basic information of that sequence.
Patsnap Bio helps you turn weeks into minutes with cutting-edge AI-enabled tools built to master the complexities of sequence retrieval and automate IP analysis with precision and ease.