TauRx Applies for UK Marketing Authorisation for HMTM to Treat Alzheimer's Disease
TauRx Pharmaceuticals Ltd, a prominent entity in tau-centric Alzheimer's disease studies, has revealed the filing of a Marketing Authorisation Application in the UK for hydromethylthionine mesylate (HMTM). This application seeks approval for the use of HMTM in treating mild cognitive impairment as well as mild to moderate dementia caused by Alzheimer's disease.
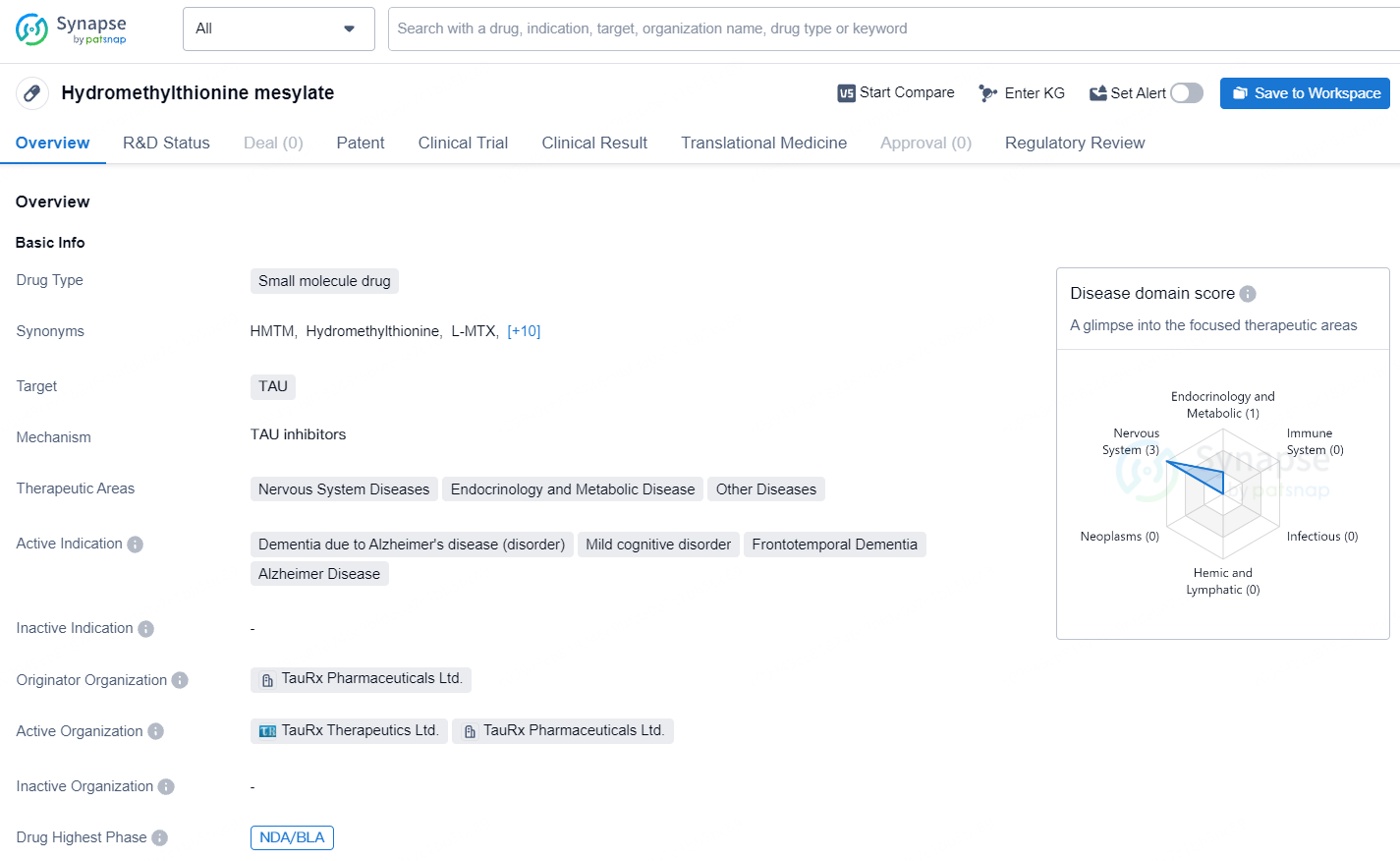
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Hydromethylthionine mesylate (HMTM) has received designation from the UK’s Medicines and Healthcare products Regulatory Agency for the Innovative Licensing and Access Pathway. Should the process succeed, the UK could become the initial country to offer patients a readily available, safe, oral treatment targeting the core tau pathology of the disease.
The Marketing Authorization Application (MAA) is anchored on comprehensive evidence from the recently concluded 24-month Phase 3 LUCIDITY trial and two prior Phase 3 studies in patients with mild to moderate Alzheimer's Disease (AD). These trials consistently demonstrated benefits in slowing cognitive decline, improving the ability to perform daily activities, and reducing brain atrophy.
The aggregation of Tau protein is closely linked with the progression and severity of cognitive impairment, neuronal damage, and neurodegeneration typical of the disease. HMTM functions by selectively blocking tau-protein aggregation in brain neurons. Additionally, it enhances brain function through its second mode of action.
HMTM has been developed as an oral therapeutic option for AD focusing on preventing tau aggregation. It also operates through an alternative mechanism that boosts acetylcholine levels in the hippocampus. The global Phase 3 LUCIDITY trial recently concluded, and findings were showcased at the AD/PD™ 2024 Alzheimer’s & Parkinson’s Diseases Conference in Lisbon, Portugal, on 7 March. Involving over 3,000 participants, HMTM has exhibited a robust safety profile and could be administered with minimal burden to patients and healthcare providers.
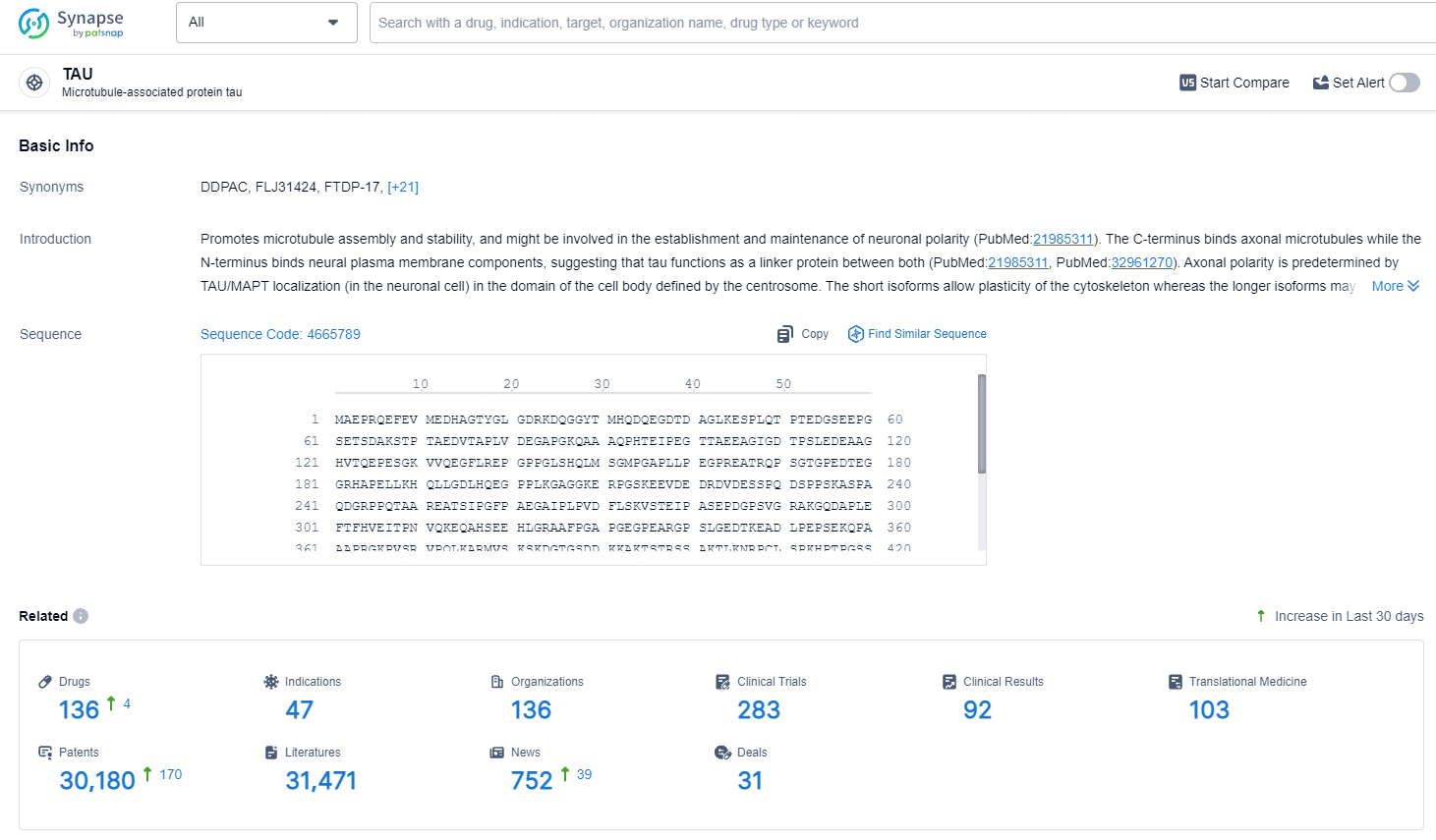
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 5, 2024, there are 136 investigational drugs for the Tau target, including 47 indications, 136 R&D institutions involved, with related clinical trials reaching 283, and as many as 30180 patents.
Hydromethylthionine mesylate shows promise as a potential treatment for various neurodegenerative and metabolic diseases, particularly in the context of Alzheimer's disease and related dementias. Its advancement to the highest phases of development both globally and in China reflects the significant progress made in its clinical evaluation. The regulatory designations further underscore the potential impact of Hydromethylthionine mesylate in addressing unmet medical needs within the pharmaceutical landscape.