Is Oliceridine approved by the FDA?
Yes, oliceridine (Olinvyk) is an FDA-approved intravenous opioid analgesic that offers a new option for managing severe acute pain. Approved on August 7, 2020, it is intended for patients who have not found relief with other treatments.
What is Oliceridine?
Oliceridine is an opioid analgesic used for the management of severe acute pain when other treatments are insufficient. It belongs to the drug class of opioids (narcotic analgesics) and is administered intravenously.
How Oliceridine Works
Oliceridine works by binding to the mu-opioid receptor in the brain, altering the perception of pain and emotional response to pain. Unlike traditional opioids, oliceridine is designed to provide effective pain relief with potentially fewer side effects, such as respiratory depression.
Administration and Dosage
Oliceridine is administered intravenously, usually through an infusion pump that allows for continuous dosing. The recommended dosing regimen is as follows:
Initial Dose:
- 1.5 mg intravenously
Supplemental Dose:
- 0.75 mg every hour as needed, starting 1 hour after the initial dose
Maximum Dose:
- Single dose: 3 mg
- Daily dose: 27 mg
For patient-controlled analgesia (PCA), the recommended demand dose is 0.35 mg with a 6-minute lockout period, with a maximum of 27 mg per day.
Potential Side Effects
Oliceridine can cause various side effects, ranging from common to severe:
Common Side Effects:
- Nausea
- Vomiting
- Constipation
- Headache
- Dizziness
- Itching
- Low oxygen levels in the blood
Serious Side Effects:
- Noisy or shallow breathing, sleep apnea
- Slow heart rate, weak pulse
- Light-headedness, potential fainting
- Severe constipation
- Low red blood cells (anemia)
- Low cortisol levels
- Low oxygen levels in the blood, leading to confusion, restlessness, or blue-colored lips/skin
Warnings and Precautions
Oliceridine carries the risk of addiction, overdose, and death, especially when misused or combined with alcohol and other sedatives. Other important precautions include:
- Do not use if allergic to oliceridine, or if you have severe asthma, breathing problems, or a bowel obstruction.
- Use during pregnancy can cause life-threatening withdrawal symptoms in the newborn.
- Long-term use may affect fertility in both men and women.
- Inform your doctor about any history of liver disease, respiratory disorders, low blood pressure, or any conditions affecting the brain, gallbladder, or pancreas.
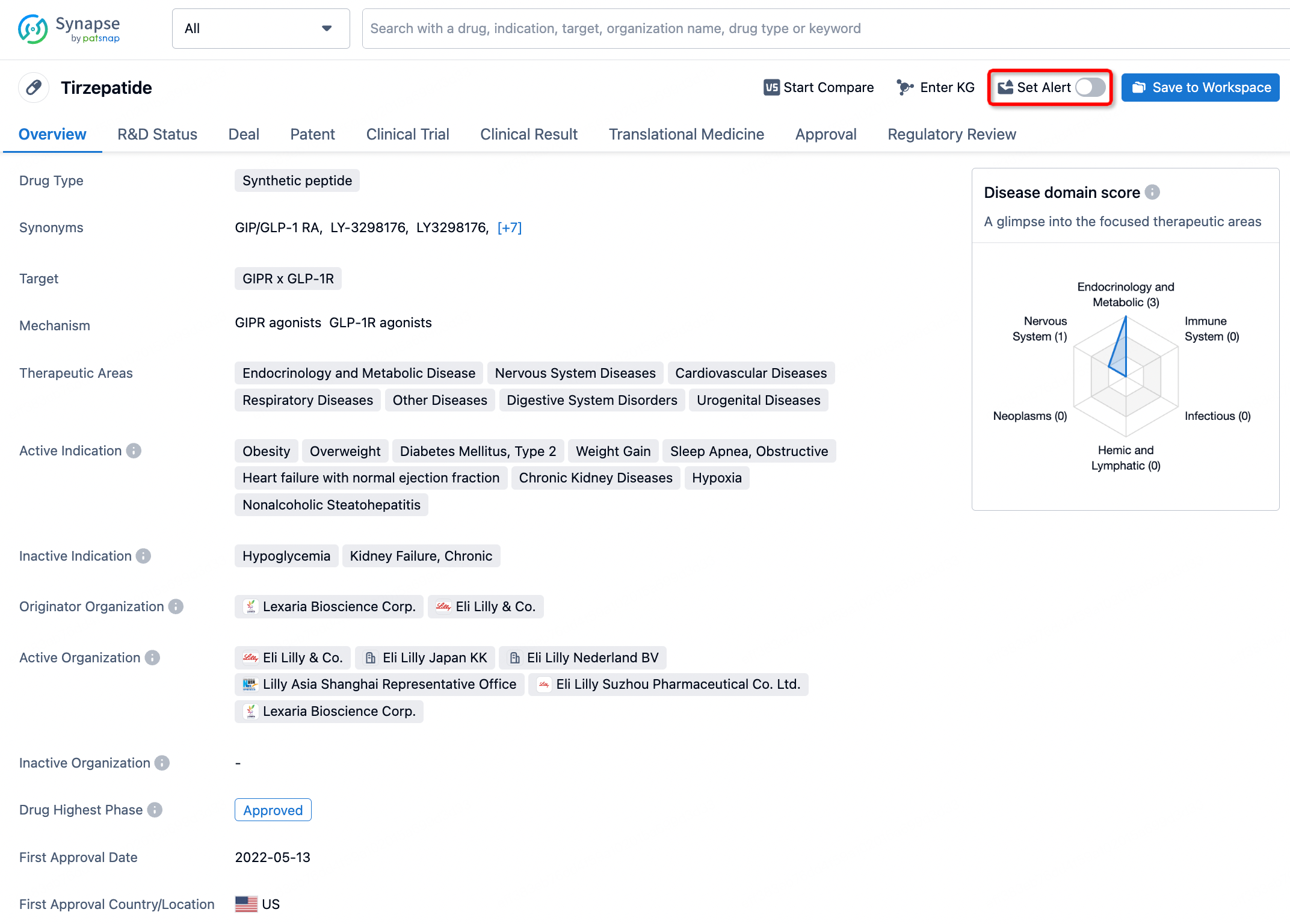
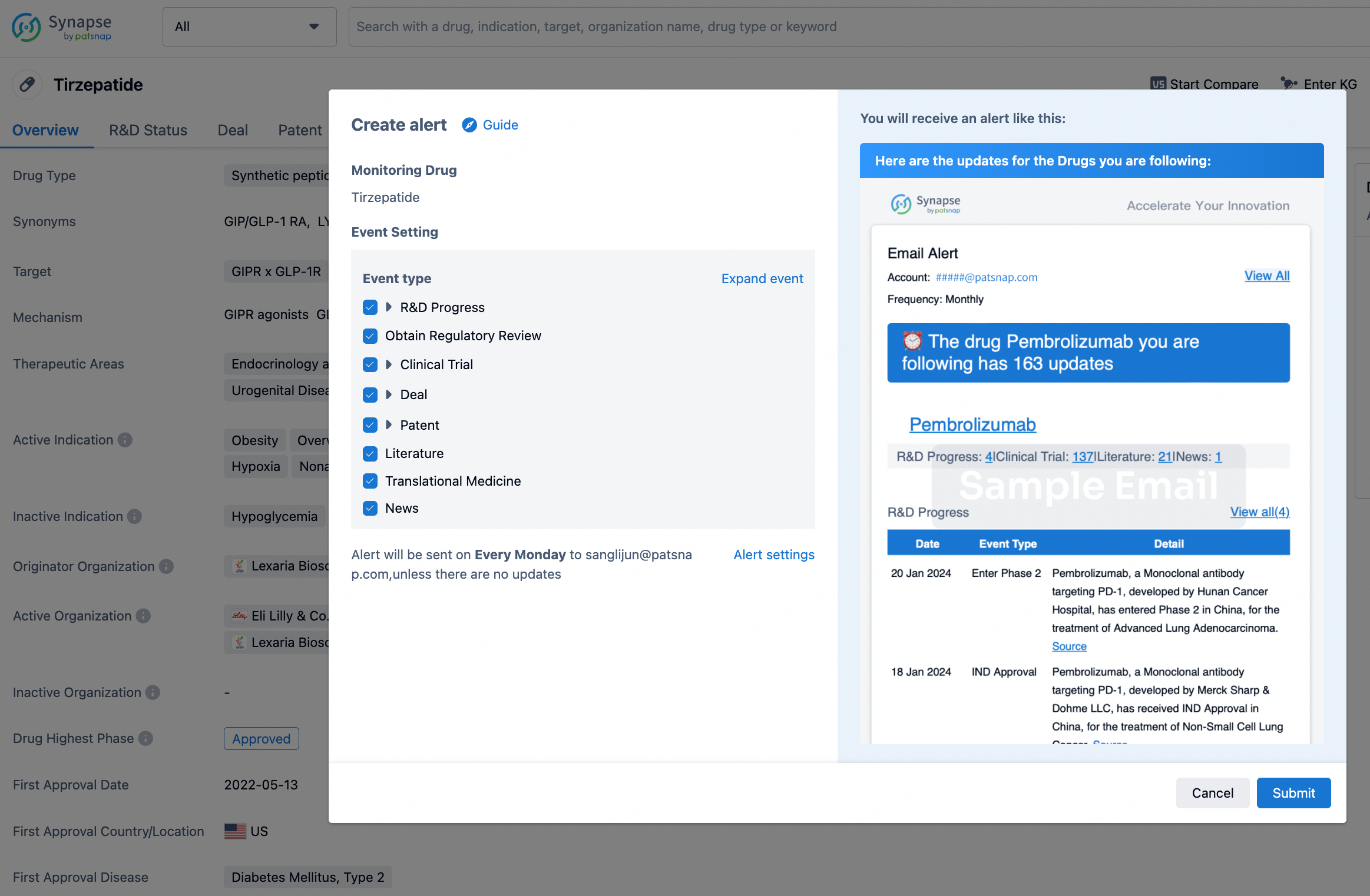
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!